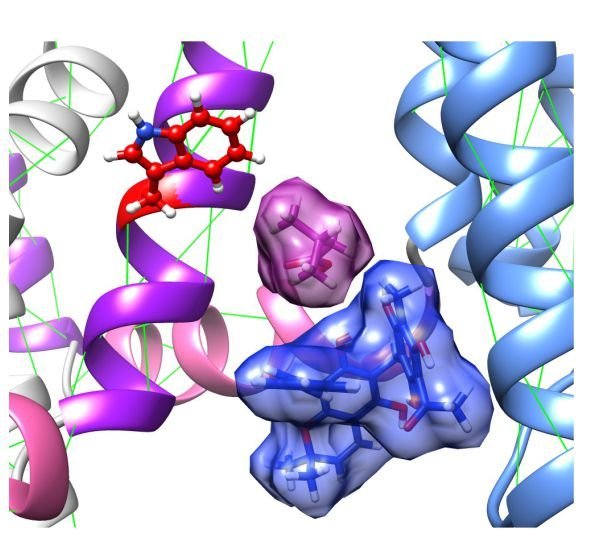
A computer model of two herbal ingredients, isovaleric acid molecules (purple, top left) and mallotoxin (blue, bottom right), occupying adjacent binding sites to synergistically activate KCNQ2/3 channels. Credit: Dr. Jeff Abbott, University of California, Arkansas for Medical Sciences
× close
A computer model of two herbal ingredients, isovaleric acid molecules (purple, top left) and mallotoxin (blue, bottom right), occupying adjacent binding sites to synergistically activate KCNQ2/3 channels. Credit: Dr. Jeff Abbott, University of California, Arkansas for Medical Sciences
Researchers in the Department of Physiology and Biophysics at the University of California, Irvine School of Medicine have discovered the molecular basis for the therapeutic action of an ancient herbal medicine used across Africa to treat a variety of illnesses, including epilepsy.
A herbal medicine extracted from the leaves of a shrub Mallotus japonicusIt was previously known that it had a seizure-suppressing effect, but the mechanism by which this occurred was unknown. Nature Communicationsfound that two components of a Chenopodium album leaf extract activate KCNQ2/3, a potassium ion channel essential for controlling electrical activity in the brain. While the two ingredients were somewhat effective on their own, when combined they were highly effective at activating KCNQ2/3 channels and preventing life-threatening seizures.
The UCI research team, consisting of postdoctoral researcher Leanne Manville, PhD, and principal investigator Jeffrey Abbott, PhD, screened individual compounds from leaf extracts for channel-opening activity and then combined the two most active compounds to discover a therapeutic synergy found in African folk medicine used for centuries. Surprisingly, one of the two compounds identified, isovaleric acid, is also the main component of valerian root, an herb used as a sleep aid for insomnia in ancient Greece and as an anticonvulsant by the British and Native Americans for centuries. Valerian root is still used by up to two million people in the United States every week as an herbal remedy for anxiety and insomnia.
“We are very interested in bringing a molecular approach to ethnobotany (the study of plants and their use by local people) to discover the molecular mechanisms of ancient remedies and use this knowledge to make safer and more effective medicines. The KCNQ channels we study are normally opened by electrical activity, but we know they are very sensitive to the presence of small molecules such as neurotransmitters, but also external molecules such as drugs, ingredients of foods or herbal extracts,” says Abbott. “Some folk remedies are at risk of being lost because traditional practices are being forgotten or the plant species used are endangered. Species loss can occur due to over-collection, habitat destruction or climate change. We are in a race against time to prevent this amazing resource from being lost forever.”
The UCI team found that the herbal extracts they studied had a different channel subtype preference than modern drugs that activate KCNQ2/3 channels, such as the anticonvulsant retigabine. Because of this, by combining the herbal compounds with retigabine, they were able to completely lock the channels open, a feat that had not previously been achieved.
“Locking the channel and keeping it open is a neat trick, but it also has clinical implications: retigabine was pulled from the market last year because of a surprising side effect: it turned the skin and the whites of the eyes blue. But we found that by combining retigabine with herbal ingredients, we could drastically reduce the dosage of retigabine needed for activity. This kind of strategy might mean that in the future we can use drugs like retigabine at doses that are low enough to be safe, while maintaining or even enhancing their efficacy by combining them with natural booster compounds derived from plants,” Abbott said.
In addition to the booster effect of herbal extracts, identifying the ability of specific chemicals within plants to activate influential ion channels such as KCNQ2/3 may in the future lead to the development of new epilepsy, anxiety and analgesic drugs that exploit the alternative chemical space offered by the molecular components of ethnoplants.
For more information:
Rían W. Manville et al, Ancient and modern anticonvulsants act synergistically at the KCNQ potassium channel binding pocket, Nature Communications (2018). Publication date: 10.1038/s41467-018-06339-2
Journal Information:
Nature Communications