1 Introduction
Tuberculosis treatment is a leading cause of drug-induced liver injury (1). The major tuberculosis drugs include ethambutol (EMB), pyrazinamide (PZA), rifampicin (RIF), and isoniazid (INH). INH, RIF, and PZA are hepatotoxic, and the combination of these drugs can further exacerbate hepatotoxicity (2). During tuberculosis treatment, 5.0–33.0% of patients experienced liver injury (3). Clinical symptoms of tuberculosis drug-induced liver injury (TBLI) include jaundice, nausea, vomiting, rash, and pruritus (1). The liver injury results in an interruption of tuberculosis treatment, hindering the treatment effect, increasing the risk of drug resistance, and in severe cases, leading to acute liver failure and even death (4, 5).
No effective treatment exists for TBLI. Nutrition is closely related to TBLI (6). Epidemiological studies indicated that body mass index (BMI) was negatively associated with the risk of TBLI (7). Randomized controlled trials indicated that carnitine, jujube syrup, and Lactobacillus casei could provide protection against TBLI (8–10). Carnitine and jujube syrup alleviated hepatocellular damage-type TBLI, which manifested as significant elevations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (8, 9). The L. casei intervention alleviated cholestasis-type TBLI (manifested as significant elevations of alkaline phosphatase and total bilirubin) by modulating the gut microbiota, reducing the blood liposaccharide content, and improving intestinal permeability (10). Animal studies reported that folic acid, vitamin B12, vitamin C, quercetin, hesperidin, curcumin, and beta-carotene could provide protection against TBLI (11–18). Nutritional interventions may be promising for mitigating TBLI (19) and other chronic diseases (20, 21).
Dietary pattern offers a holistic view of food consumption and overcomes the limitation of studying a single nutrient or food, which ignores the interactions among nutrients or foods (22, 23). Previous studies indicated that following a Mediterranean diet might lower the risk of non-alcoholic fatty liver disease (NAFLD), liver cancer, and liver fibrosis (24–26). Additionally, the healthy eating index (HEI) was negatively associated with the risk of hepatocellular carcinoma and NAFLD (27–31). The Western diet increased the risk of NAFLD, liver cirrhosis, and liver cancer, while the prudent diet reduced the risk of NAFLD, liver fibrosis, and cirrhosis (32–35). However, the relation between dietary patterns and TBLI was rarely reported.
The objective of the study is to investigate the associations of both a priori– and a posteriori-derived dietary patterns with TBLI. The China Healthy Diet Index (CHDI) was used in the study of a prior dietary pattern, which was developed based on the Dietary Guidelines for Chinese Residents (2016) in reference to the HEI-2010 (36). The CHDI was able to differentiate the diet quality of 55,528 participants from the Chinese nutrition and health surveillance (2010–2012) and was associated with a decreased risk of tuberculosis and hypertension (36, 37). Principal component analysis (PCA) was used to extract a posteriori-derived dietary pattern, which was commonly used in the nutrition literature (38, 39).
2 Materials and methods
2.1 Ethics
The study was approved by the Ethic Committee of Qingdao Center for Disease Control and Prevention (No. 2009-4). The study was conducted in accordance with the Declaration of Helsinki, and all participants provided informed consent. The trial was registered with the Chinese Clinical Trial Registry with the number ChiCTR-OCC-10000994.
2.2 Study design and population
The study was conducted at two hospitals in Shandong Province, China between 2011 and 2013. The inclusion criteria included being more than 18 years old and being newly diagnosed with pulmonary tuberculosis based on clinical symptoms, sputum smears, and computed tomography scans according to the “Technical Guidelines for Tuberculosis Prevention and Control in China” (40). The exclusion criteria included drug-resistant tuberculosis; concurrent liver, gastrointestinal, cardiovascular, respiratory diseases, cancer, human immunodeficiency virus (HIV), or mental disorders; pregnancy or nursing; AST and ALT levels above 40 U/L; and use of nutritional supplements during the past 2 months.
2.3 Procedure
The patients’ height and weight were measured upon admission to the hospital. The demographic information, which included sex, age, previous history of liver disease, diabetes status, education level, and outdoor exercise, was collected using a standard questionnaire. To assess participants’ dietary intake, a semi-quantitative food frequency questionnaire (FFQ) was conducted at the end of the second month of the tuberculosis treatment. The FFQ was previously validated (41). The FFQ included white flour, white rice, millet, corn, bran, dark vegetables, light vegetables, fruit, beef, pork, organ meat, lamb, tofu, beans, soybean milk, vegetable oil, animal oil, eggs, chicken, duck, fish, shrimp, potatoes, sweet potatoes, taro, yam, tea, dairy products, liquor, and beer. The frequency and amount of food consumption were surveyed. Food consumption was estimated in the unit of Liang (equivalent to 50 g). The consumption of tea and beer was estimated by cups. The food items were classified into 17 food groups, which included whole cereals, refined cereals, vegetables, fruit, red meat, organ meat, legumes, vegetable oil, animal oil, tea, fish and other seafood, eggs, tubers, liquor, beer, dairy products, and poultry according to the Dietary Guidelines for Chinese Residents (2016).
All patients received a standard tuberculosis treatment, which consisted of a 2-month intensive phase using EMB, PZA, RIF, and INH and a 4-month continuation phase with RIF and INH. The hospital personnel routinely tested the ALT, AST, and albumin (ALB) levels at 0, 1, 2, and 6 months after commencing the medication. An ALT or AST level greater than twice the upper limit of normal (ULN) indicated liver injury (42), while an ALT or AST level above the ULN indicated liver dysfunction. The ULNs of ALT and AST are 40 U/L (43).
2.4 Statistical analysis
The PCA with varimax rotation was used to extract the population-specific dietary patterns from 17 food groups. The analysis was tested using the Bartlett test of sphericity (p < 0.0001) and the Kaiser–Mayer–Olkin test (0.64, Supplementary Table S1). The dietary patterns with an eigenvalue ≥1.5 were extracted. The factor loadings reflect the magnitude of the relation between food groups and dietary patterns. The adherence score for each dietary pattern was calculated by the intake of each food group and the corresponding factor loadings. The CHDI was previously validated (36). The food intake was converted to per 1,000 kcal for scoring. The CHDI contains 13 items, including food variety, whole grain, refined grain, total vegetables, dark green and orange vegetables, dry bean and tuber, soybean, fruit, fish, dairy, meat and egg, shellfish and mollusk, sodium and empty calories, and calories from solid fats (SoFAS). The CHDI score ranges from 0 to 100. A higher score indicated a higher diet quality.
Statistical Package for the Social Sciences (SPSS) 26.0 was used for the statistical analysis. Inter-group differences were tested by a χ2 test (for proportions) or an ANOVA test (for continuous variables). The correlation was analyzed by Spearman’s correlation. A logistic regression analysis was used to explore the relationship between dietary patterns and the risk of liver injury and dysfunction. For each dietary pattern, the lowest tertile was used as the reference group. Age, sex, area, BMI, energy intake, and diabetes were adjusted as covariates. A p-value of <0.05 was considered indicative of statistical significance.
3 Results
3.1 A priori and a posteriori dietary patterns by CHDI and PCA
A total of 706 tuberculosis patients were recruited into the study (flow chart shown in Supplementary Figure S1). Among them, participants were excluded from the analysis due to no or incomplete FFQ (n = 38), extreme energy intake, no liver function results (n = 13), or changing treatment plan (n = 50). During the tuberculosis treatment of the participants, 49 cases of liver injury and 141 cases of liver dysfunction were identified.
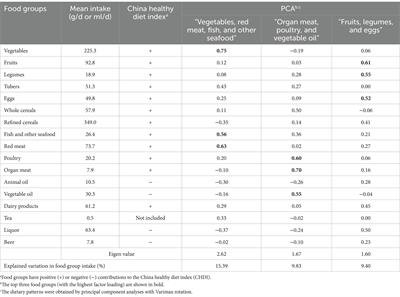
Three main dietary patterns with an eigenvalue above 1.5 were extracted by PCA (Supplementary Figure S2). These three dietary patterns accounted for 34.62% of the total variations in food intake (Table 1). The first dietary pattern, labeled “Vegetables, red meat, fish, and other seafood,” was characterized by a high intake of vegetables, red meat, fish, other seafood, tubers, and tea and a low intake of liquor, refined cereals, and animal oil. The second dietary pattern, named “Organ meat, poultry, and vegetable oil,” was characterized by a high intake of organ meat, poultry, vegetable oil, and whole cereals and a low intake of animal oil and liquor. The third dietary pattern, labeled “Fruit, legumes, and eggs,” was characterized by a high intake of fruit, legumes, eggs, liquor, dairy products, and refined cereals.
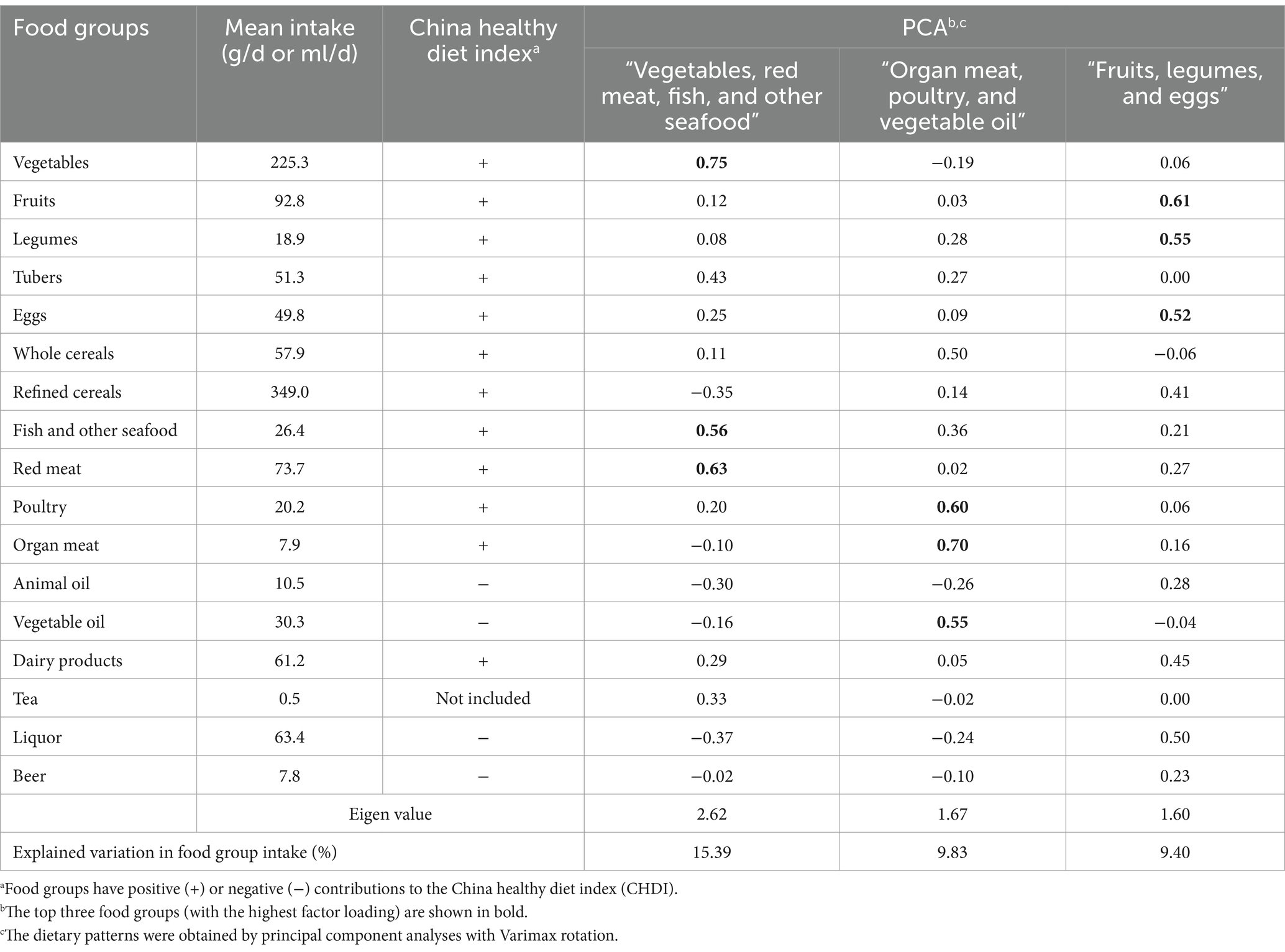
Table 1. Characteristics of a priori– and a posteriori-derived dietary pattern identified among tuberculosis patients.
We performed the Spearman correlation analysis between the dietary pattern score and nutrient intakes (Table 2). The CHDI was positively correlated with vitamin C (r = 0.66), animal protein (r = 0.65), niacin (r = 0.58), vitamin A (r = 0.57), riboflavin (r = 0.55), Ca (r = 0.50), Zn (r = 0.49), cholesterol (r = 0.48), and K (r = 0.44). The “Vegetables, red meat, fish and other seafood” dietary pattern score was positively correlated with vitamin C (r = 0.77), animal protein (r = 0.66), vitamin A (r = 0.54), niacin (r = 0.50), cholesterol (r = 0.49), riboflavin (r = 0.42), Zn (r = 0.41), and Ca (r = 0.40) and negatively correlated with vegetable protein (r = −0.40). The “Organ meat, poultry, and vegetable oil” dietary pattern score was positively correlated with fat (r = 0.49), vitamin E (r = 0.46), riboflavin (r = 0.42), and Fe (r = 0.41). The “Fruit, liquor, and legumes” dietary pattern score was positively correlated with Ca (r = 0.74), P (r = 0.70), Se (r = 0.70), riboflavin (r = 0.65), K (r = 0.66), energy (r = 0.62), Zn (r = 0.62), dietary fiber (r = 0.59), Cu (r = 0.59), animal protein (r = 0.58), cholesterol (r = 0.55), fat (r = 0.53), thiamin (r = 0.52), niacin (r = 0.49), Na (r = 0.44), Mg (r = 0.44), Fe (r = 0.44), Mn (r = 0.44), and carbohydrate (r = 0.40).
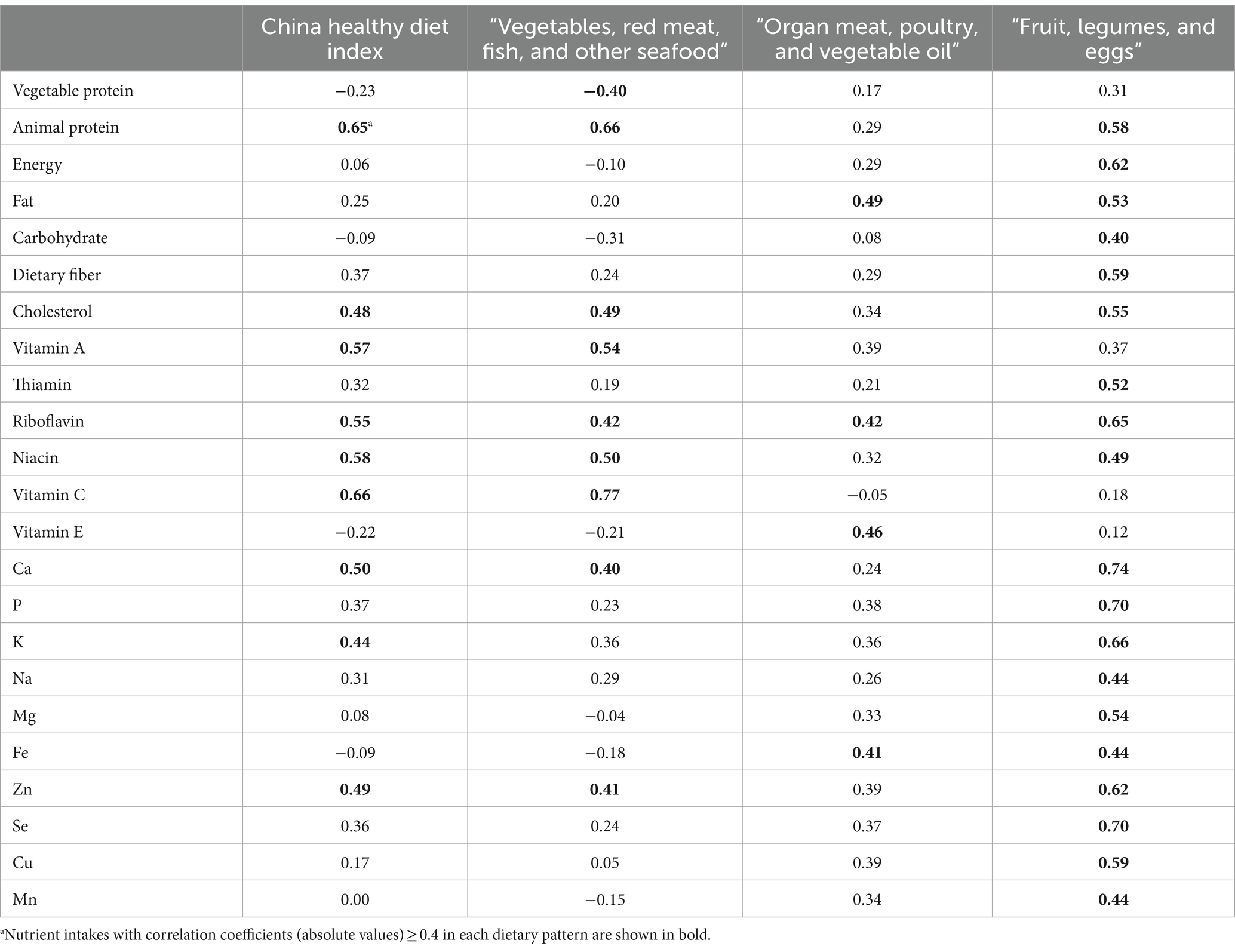
Table 2. The Spearman rank correlation coefficients between dietary pattern scores and nutrient intakes.
3.2 Baseline characteristics of the participants
The baseline characteristics of the participants are shown by tertiles of both a priori and a posteriori dietary pattern scores in Table 3. For the a priori dietary pattern, patients in the highest tertile of CHDI were younger, had a higher BMI, had more residents from the Qingdao area, and had a higher prevalence of diabetes than patients in the lowest tertile. For the a posteriori dietary pattern, patients in the highest tertile of “Vegetables, red meat, fish, and other seafood” dietary pattern score were younger, had a higher BMI, comprised more male individuals, had a higher prevalence of diabetes, and had more residents from the Qingdao area than patients in the lowest tertile. Patients in the highest tertile of “Organ meat, poultry, and vegetable oil” dietary pattern score had more residents from the Linyi area and a lower prevalence of diabetes. Patients in the highest tertile of “Fruit, legumes, and eggs” dietary pattern score had more residents from the Qingdao area and a higher prevalence of diabetes.
Table 3. Characteristics of participants (n = 605) based on the tertiles of dietary pattern scores.
3.3 Associations between dietary patterns and TBLI
The associations between dietary patterns and the risk of liver injury and liver dysfunction are shown in Tables 4, 5, respectively. For the a priori dietary pattern, the CHDI was negatively associated with the risk of liver injury [adjusted odds ratio (aOR) per SD (95% CI): 0.61 (0.40–0.94)] and liver dysfunction [aOR per SD (95% CI): 0.47 (0.35–0.64)].
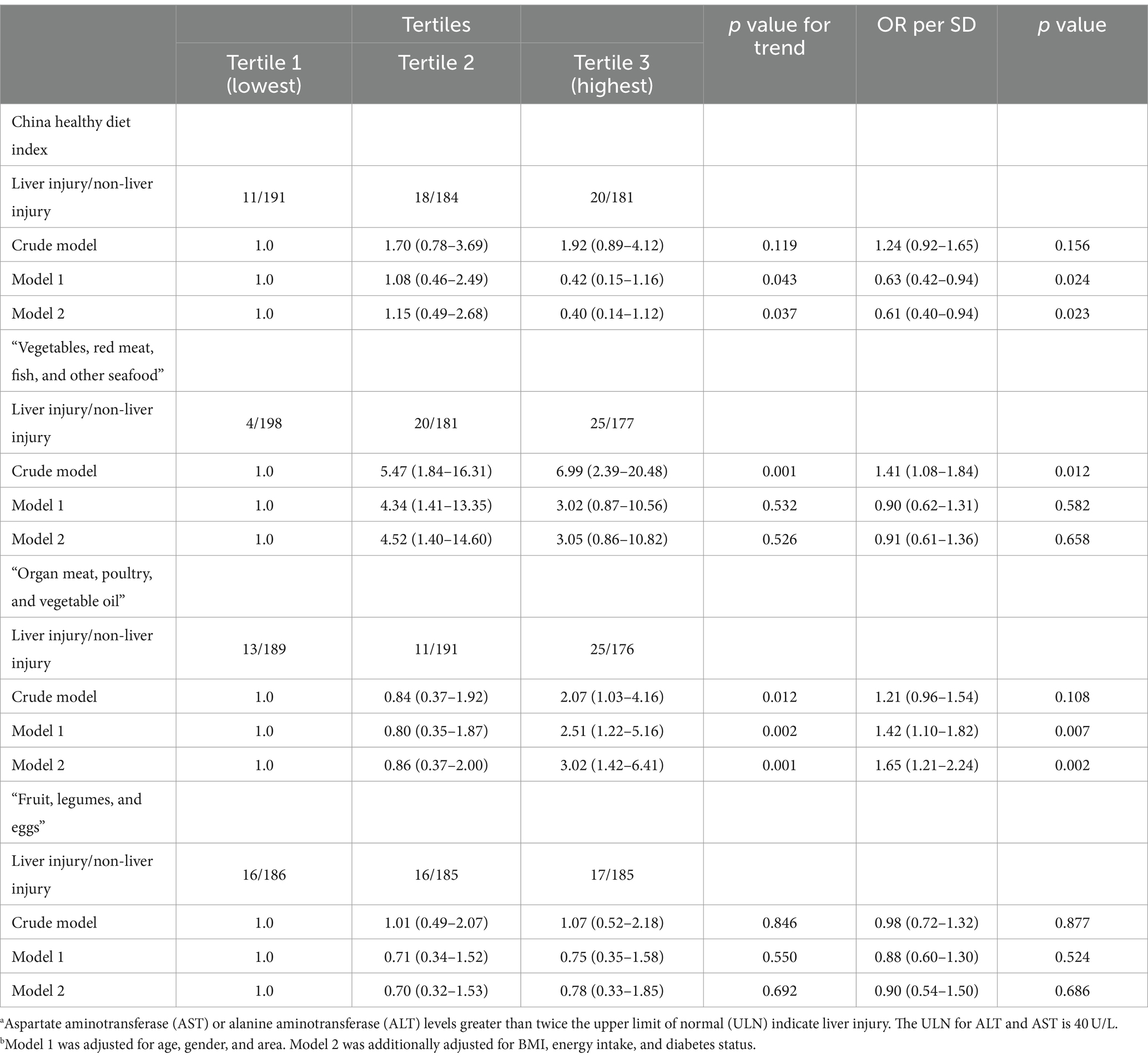
Table 4. Risk of liver injury according to the dietary pattern scores.a,b
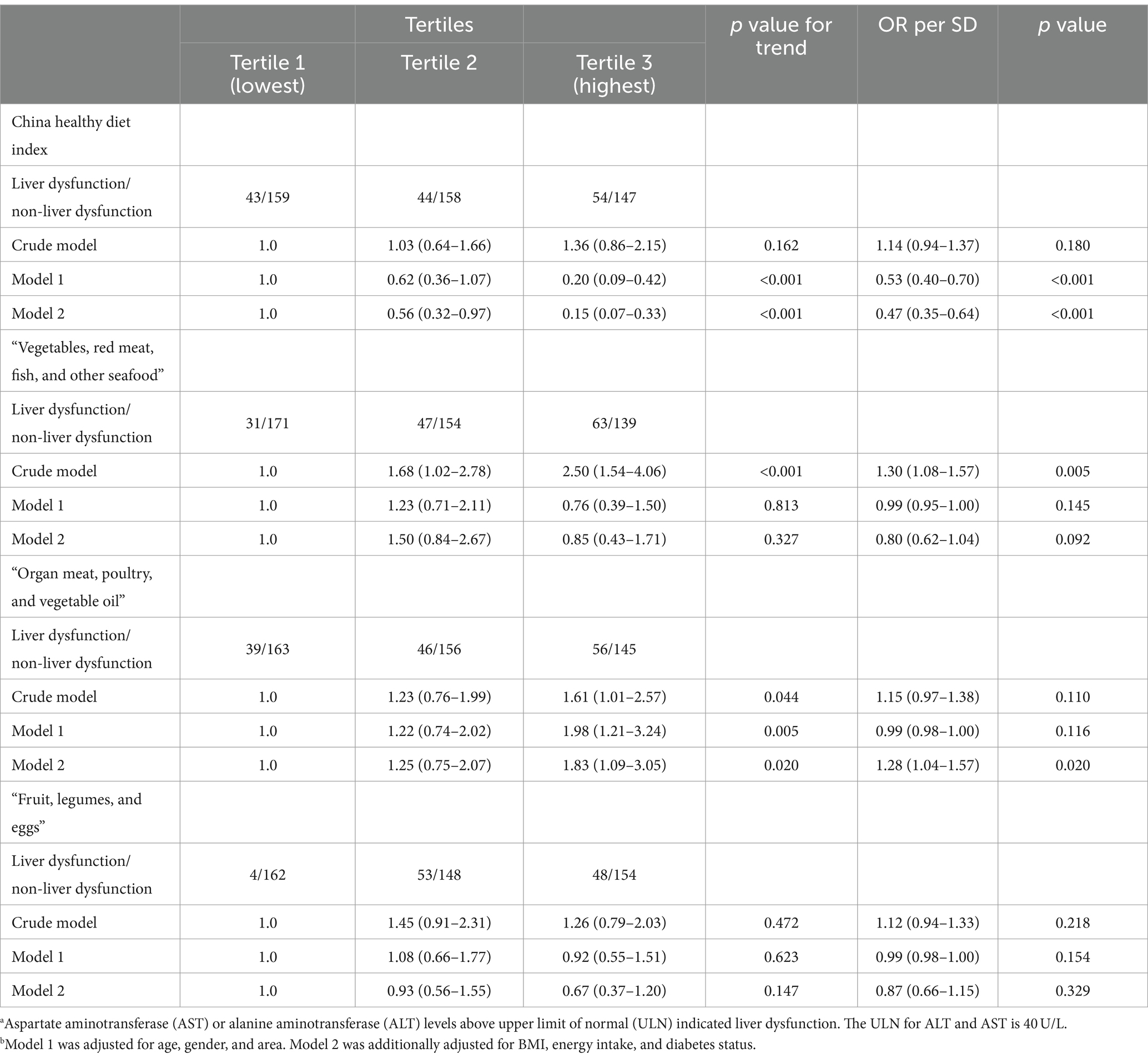
Table 5. Risk of liver dysfunction according to the dietary pattern scores.a,b
For the a posteriori dietary pattern, the patients in the highest tertile of the “Organ meat, poultry, and vegetable oil” dietary pattern score had a higher risk of liver injury [aOR (95% CI): 3.02 (1.42–6.41)] and liver dysfunction [aOR (95% CI): 1.83 (1.09–3.05)] than patients in the lowest tertile after adjusting for age, gender, area, BMI, energy intake, and diabetes status. During tuberculosis treatment, the “Vegetables, red meat, fish, and other seafood” and “Fruit, legumes, and eggs” dietary patterns were not associated with liver injury/dysfunction after adjustment for confounders.
We conducted subgroup analyses to assess whether the associations between dietary pattern scores and the risk of TBLI/tuberculosis drug-induced liver dysfunction varied by age, sex, area, BMI, diabetes, and smoking status (Supplementary Tables S2, S3). After adjusting for covariates, the negative association between the CHDI score and the risk of TBLI/tuberculosis drug-induced liver dysfunction was particularly significant in male patients, Qingdao residents, patients with a BMI of <24, and patients without diabetes or smoking. The positive association between the “Organ meat, poultry, and vegetable oil” pattern and the risk of TBLI/tuberculosis drug-induced liver dysfunction was particularly significant in male patients, younger patients (≤ 65 years), Linyi residents, patients with a BMI of <24, and patients without diabetes.
4 Discussion
To our knowledge, this study is one of the first to investigate the relation between dietary patterns and the risk of TBLI. Our results suggested that the a priori dietary pattern, based on the CHDI, was negatively associated with the risk of TBLI, while the “Organ meat, poultry, and vegetable oil” dietary pattern, extracted by PCA, was positively associated with the risk of TBLI.
Our results showed a negative relation between the CHDI and the risk of TBLI. The CHDI is developed in reference to the HEI-2010, both of which grant high scores for the high intake of whole grain, total vegetables, dark vegetables, fruit, dairy products, meat, eggs, fish, and other seafood and for the low intake of animal oil and sodium (36). Our results indicated that a balanced diet (e.g., a diet with a high CHDI score) may be beneficial for alleviating TBLI. Consistently, previous studies reported that another balanced diet, a diet with a high HEI score, was negatively associated with other liver diseases such as NAFLD and hepatocellular carcinoma (29, 31). A cohort study including 4,94,942 participants found that adherence to HEI may reduce the risk of developing hepatocellular carcinoma and the risk of dying from chronic liver disease (29). A cross-sectional study including 2,892 participants found that HEI was associated with a reduced incidence of NAFLD (31).
For the posteriori dietary patterns extracted by PCA, our results suggested that the “Organ meat, poultry, and vegetable oil” dietary pattern was positively associated with the risk of TBLI and tuberculosis drug-induced liver dysfunction. The associations became stronger after adjusting for confounders including age, gender, area, BMI, energy intake, and diabetes status. Subgroup analyses indicated that the positive associations were observed between the “Organ meat, poultry, and vegetable oil” dietary pattern and the risk of TBLI and tuberculosis drug-induced liver dysfunction were particularly significant in male patients, Linyi residents, and participants aged ≤65 years. In addition, strongly positive associations between the “Organ meat, poultry, and vegetable oil” dietary pattern and the dietary intake of total fat, riboflavin, vitamin E, and iron were observed. Consistently, the intake of vegetable oil was negatively correlated with TBLI, as indicated in our previous research (44).
Several mechanisms may explain the observed associations between dietary patterns and TBLI in our study. First, diseases such as TBLI are closely related to oxidative stress and chronic inflammation (19, 45). A higher diet quality score (such as those rated by a higher HEI or CHDI score) was associated with a reduced level of oxidative stress and inflammation biomarkers (46, 47). Thus, a diet with a higher CHDI score may alleviate TBLI by reducing oxidative stress and inflammation. Second, the major individual foods comprising the dietary patterns may contribute to the associations with TBLI. Vegetable intake was associated with a reduced risk of TBLI in our previous cohort study, which may be attributed to the phytochemicals in vegetables (44). In vitro and animal studies showed that phytochemicals could reduce free radicals and inflammation (48). On the other hand, organ meat and vegetable oil consumption were positively associated with NAFLD and TBLI, respectively (44, 49). Vegetable oil is enriched with linoleic acid, which may induce liver injury by increasing the activity of cytochrome P450 2E1 and inducing liver inflammation (50). Thus, the “Organ meat, poultry, and vegetable oil” dietary pattern, which is characterized by a high intake of organ meat, poultry, and vegetable oil, was associated with an increased risk of TBLI.
The major strengths of the current study are the following. First, we investigated the associations of both a priori and posteriori dietary patterns with TBLI, which represent a comprehensive approach. Second, in the investigation of the a priori dietary pattern, a previously validated and specifically designed index for the Chinese population, the CHDI, was used (36). Third, detailed demographic information was systematically collected during the study, which allowed us to adjust for common confounding variables associated with TBLI, including BMI, sex, age, location, energy intake, and diabetes.
The limitations should be acknowledged. First, the generalizability of the study needs future study because all the included subjects were Chinese. Second, due to the low incidence of tuberculosis (58/1,000,000 in China), the sample size was relatively small (51), which may weaken the statistical power. Third, the study was observational, and no causal relationship could be drawn. However, we carefully adjusted for common confounding factors.
In conclusion, a higher CHDI score was associated with a reduced risk of TBLI, while the “Organ meat, poultry, vegetable oil” dietary pattern, which was rich in organ meat, poultry, and vegetable oil and low in vegetables, was positively associated with the risk of TBLI. A diet with a high CHDI score and involving less organ meat and vegetable oil may be recommended during tuberculosis treatment to prevent TBLI. Future studies may validate this conclusion in a non-Chinese population with a larger sample size.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethic Committee of Qingdao Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JW: Funding acquisition, Writing – original draft, Data curation, Formal analysis. YZ: Writing – original draft, Data curation, Formal analysis. CZ: Investigation, Writing – review & editing. KX: Writing – review & editing. YL: Investigation, Writing – review & editing. SZ: Investigation, Writing – review & editing. AM: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82003446) and the World Diabetes Foundation (No. WDF08-380).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1393523/full#supplementary-material
References
1. Wang, S, Shangguan, Y, Ding, C, Li, P, Ji, Z, Shao, J, et al. Risk factors for acute liver failure among inpatients with anti-tuberculosis drug-induced liver injury. J Int Med Res. (2020) 48:300060518811512. doi: 10.1177/0300060518811512
PubMed Abstract | Crossref Full Text | Google Scholar
3. Saukkonen, JJ, Cohn, DL, Jasmer, RM, Schenker, S, Jereb, JA, Nolan, CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. (2006) 174:935–52. doi: 10.1164/rccm.200510-1666ST
Crossref Full Text | Google Scholar
4. Zheng, J, Guo, MH, Peng, HW, Cai, XL, Wu, YL, and Peng, XE. The role of hepatitis B infection in anti-tuberculosis drug-induced liver injury: a meta-analysis of cohort studies. Epidemiol Infect. (2020) 148:e290. doi: 10.1017/s0950268820002861
PubMed Abstract | Crossref Full Text | Google Scholar
5. Singh, J, Garg, PK, and Tandon, RK. Hepatotoxicity due to antituberculosis therapy. Clinical profile and reintroduction of therapy. J Clin Gastroenterol. (1996) 22:211–4. doi: 10.1097/00004836-199604000-00012
Crossref Full Text | Google Scholar
6. Martin, SJ, and Sabina, EP. Malnutrition and associated disorders in tuberculosis and its therapy. J Diet Suppl. (2019) 16:602–10. doi: 10.1080/19390211.2018.1472165
Crossref Full Text | Google Scholar
7. Ali, N, Gupta, N, and Saravu, K. Malnutrition as an important risk factor for drug-induced liver injury in patients on anti-tubercular therapy: an experience from a tertiary care center in South India. Drug Discov Ther. (2020) 14:135–8. doi: 10.5582/ddt.2020.03029
PubMed Abstract | Crossref Full Text | Google Scholar
8. Maddahi, SZ, Jokar, A, Kamalinejad, M, and Behnampur, N. The efficacy of jujube syrup on the prevention of drug-induced hepatotoxicity in pulmonary tuberculosis patients: a pilot randomized double-blind placebo-controlled clinical trial. Pharmacol Res Perspect. (2022) 10:e00902. doi: 10.1002/prp2.902
PubMed Abstract | Crossref Full Text | Google Scholar
9. Hatamkhani, S, Khalili, H, Karimzadeh, I, Dashti-Khavidaki, S, Abdollahi, A, and Jafari, S. Carnitine for prevention of antituberculosis drug-induced hepatotoxicity: a randomized, clinical trial. J Gastroenterol Hepatol. (2014) 29:997–1004. doi: 10.1111/jgh.12474
PubMed Abstract | Crossref Full Text | Google Scholar
10. Xiong, K, Cai, J, Liu, P, Wang, J, Zhao, S, Xu, L, et al. Lactobacillus casei alleviated the abnormal increase of cholestasis-related liver indices during tuberculosis treatment: a post hoc analysis of randomized controlled trial. Mol Nutr Food Res. (2021) 65:e2100108. doi: 10.1002/mnfr.202100108
PubMed Abstract | Crossref Full Text | Google Scholar
11. Jiang, L, Gai, X, Ni, Y, Qiang, T, Zhang, Y, Kang, X, et al. Folic acid protects against tuberculosis-drug-induced liver injury in rats and its potential mechanism by metabolomics. J Nutr Biochem. (2023) 112:109214. doi: 10.1016/j.jnutbio.2022.109214
PubMed Abstract | Crossref Full Text | Google Scholar
12. Joseph Martin, S, and Evan, PS. Comparative modulation of levels of oxidative stress in the liver of anti-tuberculosis drug treated wistar rats by vitamin B12, beta-carotene, and spirulina fusiformis: role of NF-κB, iNOS, IL-6, and IL-10. J Cell Biochem. (2017) 118:3825–33. doi: 10.1002/jcb.26032
PubMed Abstract | Crossref Full Text | Google Scholar
13. Ergul, Y, Erkan, T, Uzun, H, Genc, H, Altug, T, and Erginoz, E. Effect of vitamin C on oxidative liver injury due to isoniazid in rats. Pediatr Int. (2010) 52:69–74. doi: 10.1111/j.1442-200X.2009.02891.x
PubMed Abstract | Crossref Full Text | Google Scholar
14. Zhang, Y, Qu, X, Gao, H, Zhai, J, Tao, L, Sun, J, et al. Quercetin attenuates NLRP3 inflammasome activation and apoptosis to protect INH-induced liver injury via regulating SIRT1 pathway. Int Immunopharmacol. (2020) 85:106634. doi: 10.1016/j.intimp.2020.106634
PubMed Abstract | Crossref Full Text | Google Scholar
15. Li, Y, Luo, WW, Cheng, X, Xiang, HR, He, B, Zhang, QZ, et al. Curcumin attenuates isoniazid-induced hepatotoxicity by upregulating the SIRT1/PGC-1α/NRF1 pathway. J Appl Toxicol. (2022) 42:1192–204. doi: 10.1002/jat.4288
PubMed Abstract | Crossref Full Text | Google Scholar
16. He, L, Guo, Y, Deng, Y, Li, C, Zuo, C, and Peng, W. Involvement of protoporphyrin IX accumulation in the pathogenesis of isoniazid/rifampicin-induced liver injury: the prevention of curcumin. Xenobiotica. (2017) 47:154–63. doi: 10.3109/00498254.2016.1160159
PubMed Abstract | Crossref Full Text | Google Scholar
17. Zhang, G, Zhu, J, Zhou, Y, Wei, Y, Xi, L, Qin, H, et al. Hesperidin alleviates oxidative stress and upregulates the multidrug resistance protein 2 in isoniazid and rifampicin-induced liver injury in rats. J Biochem Mol Toxicol. (2016) 30:342–9. doi: 10.1002/jbt.21799
Crossref Full Text | Google Scholar
18. Jiang, L, Ni, Y, Zhao, C, Gao, D, Gai, X, Xiong, K, et al. Folic acid protects against isoniazid-induced liver injury via the m6A RNA methylation of cytochrome P450 2E1 in mice. Front Nutr. (2024) 11:1389684. doi: 10.3389/fnut.2024.1389684
PubMed Abstract | Crossref Full Text | Google Scholar
19. Fu, Y, Du, X, Cui, Y, Xiong, K, and Wang, J. Nutritional intervention is promising in alleviating liver injury during tuberculosis treatment: a review. Front Nutr. (2023) 10:1261148. doi: 10.3389/fnut.2023.1261148
PubMed Abstract | Crossref Full Text | Google Scholar
20. Keramati, M, Kheirouri, S, Musazadeh, V, and Alizadeh, M. Association of high dietary acid load with the risk of cancer: a systematic review and meta-analysis of observational studies. Front Nutr. (2022) 9:816797. doi: 10.3389/fnut.2022.816797
PubMed Abstract | Crossref Full Text | Google Scholar
21. Musazadeh, V, Kavyani, Z, Naghshbandi, B, Dehghan, P, and Vajdi, M. The beneficial effects of omega-3 polyunsaturated fatty acids on controlling blood pressure: an umbrella meta-analysis. Front Nutr. (2022) 9:985451. doi: 10.3389/fnut.2022.985451
PubMed Abstract | Crossref Full Text | Google Scholar
22. Barbaresko, J, Koch, M, Schulze, MB, and Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. (2013) 71:511–27. doi: 10.1111/nure.12035
Crossref Full Text | Google Scholar
23. Schwingshackl, L, Schwedhelm, C, Galbete, C, and Hoffmann, G. Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. (2017) 9:1063. doi: 10.3390/nu9101063
PubMed Abstract | Crossref Full Text | Google Scholar
24. Haigh, L, Kirk, C, El Gendy, K, Gallacher, J, Errington, L, Mathers, JC, et al. The effectiveness and acceptability of mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Clin Nutr. (2022) 41:1913–31. doi: 10.1016/j.clnu.2022.06.037
Crossref Full Text | Google Scholar
25. Kouvari, M, Boutari, C, Chrysohoou, C, Fragkopoulou, E, Antonopoulou, S, Tousoulis, D, et al. Mediterranean diet is inversely associated with steatosis and fibrosis and decreases ten-year diabetes and cardiovascular risk in NAFLD subjects: results from the Attica prospective cohort study. Clin Nutr. (2021) 40:3314–24. doi: 10.1016/j.clnu.2020.10.058
PubMed Abstract | Crossref Full Text | Google Scholar
26. Turati, F, Trichopoulos, D, Polesel, J, Bravi, F, Rossi, M, Talamini, R, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol. (2014) 60:606–11. doi: 10.1016/j.jhep.2013.10.034
Crossref Full Text | Google Scholar
27. Kalafati, IP, Borsa, D, Dimitriou, M, Revenas, K, Kokkinos, A, and Dedoussis, GV. Dietary patterns and non-alcoholic fatty liver disease in a Greek case-control study. Nutrition. (2019) 61:105–10. doi: 10.1016/j.nut.2018.10.032
PubMed Abstract | Crossref Full Text | Google Scholar
28. George, ES, Sood, S, Broughton, A, Cogan, G, Hickey, M, Chan, WS, et al. The association between diet and hepatocellular carcinoma: a systematic review. Nutrients. (2021) 13:172. doi: 10.3390/nu13010172
PubMed Abstract | Crossref Full Text | Google Scholar
29. Li, WQ, Park, Y, McGlynn, KA, Hollenbeck, AR, Taylor, PR, Goldstein, AM, et al. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology. (2014) 60:588–97. doi: 10.1002/hep.27160
PubMed Abstract | Crossref Full Text | Google Scholar
30. Chen, PY, Fang, AP, Wang, XY, Lan, QY, Liao, GC, Liu, ZY, et al. Adherence to the Chinese or American dietary guidelines is associated with a lower risk of primary liver cancer in China: a case-control study. Nutrients. (2018) 10:1113. doi: 10.3390/nu10081113
PubMed Abstract | Crossref Full Text | Google Scholar
31. Heredia, NI, Zhang, X, Balakrishnan, M, Daniel, CR, Hwang, JP, McNeill, LH, et al. Physical activity and diet quality in relation to non-alcoholic fatty liver disease: a cross-sectional study in a representative sample of U.S. adults using NHANES 2017-2018. Prev Med. (2022) 154:106903. doi: 10.1016/j.ypmed.2021.106903
Crossref Full Text | Google Scholar
32. Hassani Zadeh, S, Mansoori, A, and Hosseinzadeh, M. Relationship between dietary patterns and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2021) 36:1470–8. doi: 10.1111/jgh.15363
PubMed Abstract | Crossref Full Text | Google Scholar
33. Guo, W, Ge, X, Lu, J, Xu, X, Gao, J, Wang, Q, et al. Diet and risk of non-alcoholic fatty liver disease, cirrhosis, and liver cancer: a large prospective cohort study in UK biobank. Nutrients. (2022) 14:5335. doi: 10.3390/nu14245335
PubMed Abstract | Crossref Full Text | Google Scholar
34. Oddy, WH, Herbison, CE, Jacoby, P, Ambrosini, GL, O’Sullivan, TA, Ayonrinde, OT, et al. The western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. (2013) 108:778–85. doi: 10.1038/ajg.2013.95
PubMed Abstract | Crossref Full Text | Google Scholar
35. Soleimani, D, Ranjbar, G, Rezvani, R, Goshayeshi, L, Razmpour, F, and Nematy, M. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes. (2019) 12:315–24. doi: 10.2147/dmso.S198744
PubMed Abstract | Crossref Full Text | Google Scholar
36. He, Y, Fang, Y, Yang, X, and Ding, G. Establishment and application of China healthy diet index. Acta Nutr Sin. (2017) 39:436–41. doi: 10.13325/j.cnki.acta.nutr.sin.2017.05.011
Crossref Full Text | Google Scholar
37. Ji, F, Yang, Y, Xu, L, Cai, J, Ni, M, Wang, Q, et al. Poor diet quality evaluated with the China healthy diet index in Chinese tuberculosis patients. J Hum Nutr Diet. (2022) 35:1192–201. doi: 10.1111/jhn.12985
PubMed Abstract | Crossref Full Text | Google Scholar
38. Steck, SE, and Murphy, EA. Dietary patterns and cancer risk. Nat Rev Cancer. (2020) 20:125–38. doi: 10.1038/s41568-019-0227-4
Crossref Full Text | Google Scholar
39. Lever, J, Krzywinski, M, and Altman, N. Principal component analysis. Nat Methods. (2017) 14:641–2. doi: 10.1038/nmeth.4346
Crossref Full Text | Google Scholar
40. Mingting, C, Yanlin, Z, Caihong, X, and Hui, Z. Technical Guidelines for Tuberculosis Prevention and Control in China. Beijing: People’s Medical Publishing House (2021).
Google Scholar
41. Lin, S, Gao, T, Sun, C, Jia, M, Liu, C, Ma, X, et al. Association of dietary patterns and endoscopic gastric mucosal atrophy in an adult Chinese population. Sci Rep. (2019) 9:16567. doi: 10.1038/s41598-019-52951-7
PubMed Abstract | Crossref Full Text | Google Scholar
42. Chalasani, NP, Maddur, H, Russo, MW, Wong, RJ, and Reddy, KR. ACG clinical guideline: diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. (2021) 116:878–98. doi: 10.14309/ajg.0000000000001259
PubMed Abstract | Crossref Full Text | Google Scholar
43. Wan, X, and Lu, X. Diagnostics. Beijing, China: People’s Medical Publishing House (2018).
Google Scholar
44. Wang, J, Xiong, K, Xu, L, Zhang, C, Zhao, S, Liu, Y, et al. Dietary intake of vegetables and cooking oil was associated with drug-induced liver injury during tuberculosis treatment: a preliminary cohort study. Front Nutr. (2021) 8:652311. doi: 10.3389/fnut.2021.652311
PubMed Abstract | Crossref Full Text | Google Scholar
45. Zarezadeh, M, Barzegari, M, Aghapour, B, Adeli, S, Khademi, F, Musazadeh, V, et al. Melatonin effectiveness in amelioration of oxidative stress and strengthening of antioxidant defense system: findings from a systematic review and dose-response meta-analysis of controlled clinical trials. Clin Nutr ESPEN. (2022) 48:109–20. doi: 10.1016/j.clnesp.2022.01.038
PubMed Abstract | Crossref Full Text | Google Scholar
46. Kim, JY, Yang, YJ, Yang, YK, Oh, SY, Hong, YC, Lee, EK, et al. Diet quality scores and oxidative stress in Korean adults. Eur J Clin Nutr. (2011) 65:1271–8. doi: 10.1038/ejcn.2011.120
Crossref Full Text | Google Scholar
47. Fung, TT, McCullough, ML, Newby, PK, Manson, JE, Meigs, JB, Rifai, N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. (2005) 82:163–73. doi: 10.1093/ajcn.82.1.163
PubMed Abstract | Crossref Full Text | Google Scholar
48. Madrigal-Santillán, E, Madrigal-Bujaidar, E, Álvarez-González, I, Sumaya-Martínez, MT, Gutiérrez-Salinas, J, Bautista, M, et al. Review of natural products with hepatoprotective effects. World J Gastroenterol. (2014) 20:14787–804. doi: 10.3748/wjg.v20.i40.14787
PubMed Abstract | Crossref Full Text | Google Scholar
49. Li, H, Zheng, X, Sabina, R, Amrish, T, Meng, G, Zhang, Q, et al. Organ meat consumption and risk of non-alcoholic fatty liver disease: the Tianjin chronic low-grade systemic inflammation and health cohort study. Br J Nutr. (2023) 130:276–83. doi: 10.1017/s0007114522000629
PubMed Abstract | Crossref Full Text | Google Scholar
50. Nanji, AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. (2004) 34:21–5. doi: 10.1016/j.alcohol.2004.08.005
Crossref Full Text | Google Scholar