ACS-20 is a key mediator of DR-induced lifespan and healthspan extension
To characterize the roles of lipid metabolism in the anti-ageing effect of DR, we performed an RNAi-based genetic screen of 21 acyl-CoA synthetase (acs) genes for their effects on lifespan under DR conditions in C. elegans. Compared to the control RNAi, the acs-20 RNAi treatment most significantly suppresses the prolonged longevity by DR (Supplementary Fig. 1). acs-20 encodes the C. elegans ortholog of the fatty acid transporter 4 (FATP4), mutations in which leads to abnormal lipid metabolism in the epidermis and disease such as ichthyosis prematurity syndrome (IPS) in mammals21,22. In C. elegans, the acs-20 mutant shows abnormalities in cuticle structures23.
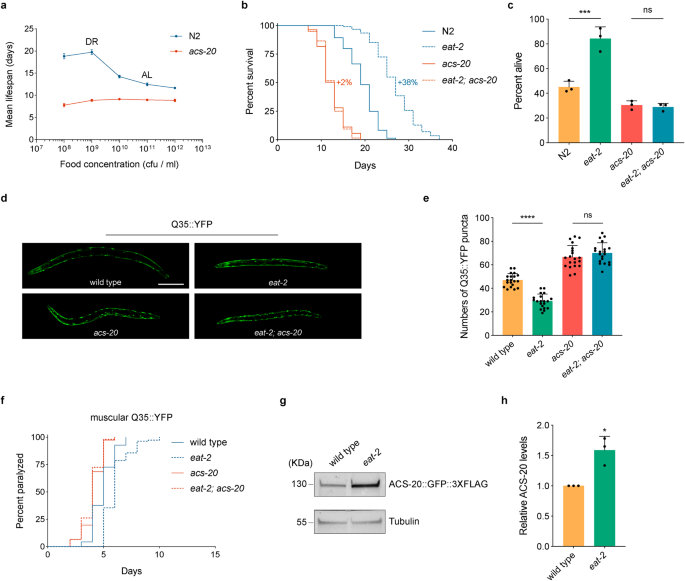
To further validate the role of ACS-20 in DR-induced lifespan extension, wild-type N2 and acs-20 deletion mutant animals were treated with bacterial food at different concentrations (1.0 × 108–1.0 × 1012 cfu/ml) from day 1 adulthood for survival assays. Unlike N2 animals, which exhibit more lifespan extension with food dilution until the optimal DR condition (1.0 × 109 cfu/ml), the acs-20 mutant shows no significant changes in lifespan under different nutrient conditions (Fig. 1a). The eat-2 mutant, which has reduced feeding and significantly extended lifespan, has been widely used as a genetic mimic of DR8. The acs-20 mutant completely abolishes the prolonged longevity of eat-2 animals (Fig. 1b). Therefore, ACS-20 is a key mediator of DR-induced longevity.
a Mean lifespan of the wild-type N2 and acs-20 mutant fed with bacterial food at different concentrations (1.0 × 108–1.0 × 1012 cfu/ml). Data are represented as mean ± SD based on three independent experiments. AL, ad libitum (1.0 × 1011 cfu/ml), DR, optimal dietary restriction condition (1.0 × 109 cfu/ml). b Survival curves of N2, eat-2, acs-20, and eat-2; acs-20 mutant animals. Percentages indicate changes in mean lifespan induced by the eat-2 mutation. N2 vs. eat-2, p < 0.0001; acs-20 vs. eat-2; acs-20, p = 0.8271 (log-rank tests). c Survival percentages of N2, eat-2, acs-20, and eat-2; acs-20 mutant animals incubated at 35 °C for 10 h. Data are represented as mean ± SD (n = 3). ***p < 0.001; ns, p = 0.9875 (One-way ANOVA with Turkey’s multiple comparisons test). d, e Representative photographs of 3-day-old adult wild-type, eat-2, acs-20 and eat-2; acs-20 mutant animals expressing the muscular Q35::YFP (d) and quantification of the Q35::YFP punctae (e). Data are represented as mean ± SD (n = 20). ****p < 0.0001; ns, p = 0.5231 (One-way ANOVA with Turkey’s multiple comparisons test). Scale bar, 100 μm. f Paralysis of wild-type, eat-2, acs-20 and eat-2; acs-20 mutant animals expressing the muscular Q35::YFP. Wild-type vs. eat-2, p < 0.0001; acs-20 vs. eat-2; acs-20, p = 0.2364 (log-rank tests). g, h Immunoblots (g) and quantification (h) of ACS-20::GFP::3✕FLAG and tubulin protein levels in the wild type and eat-2 mutant. The ratio of band intensity of ACS-20::GFP::3✕FLAG to tubulin was normalized to the wild type. Data are represented as mean ± SD (n = 3). *p = 0.0111 (two-tailed t-test). Source data are provided as a Source Data file.
In addition to lifespan, healthspan is also an important assessment of healthy ageing1. To explore the role of acs-20 in healthspan regulation, we first examined thermotolerance phenotypes of N2, eat-2, acs-20, and eat-2; acs-20 mutants by heat shock at 35 °C for 10 h. The eat-2 mutant shows significantly increased resistance to the heat stress compared to the wild-type N2, whereas the acs-20 mutation completely blocks the protective effect of eat-2 (Fig. 1c). Polyglutamine (polyQ) expansion-induced proteotoxicity has been linked with several degenerative diseases24. We took advantage of an established polyQ model, in which a transgene expresses polyQ (Q35) fused with YFP in the body wall muscle25, to test the effect of acs-20 on proteostasis. Compared to the wild type, the eat-2 mutant shows significantly less Q35::YFP aggregates and delayed paralysis due to the proteotoxicity in body wall muscle cells (Fig. 1d–f). However, the protective effect of eat-2 is abrogated by the acs-20 mutation (Fig. 1d–f). Taken together, these results demonstrate that ACS-20 is required for DR-induced healthspan extension.
To further examine whether ACS-20 is regulated by nutrients, we performed CRISPR/Cas9-based genome editing experiment to knock in GFP::3 ✕ FLAG coding sequence to the 3′ end of acs-20. We then compared ACS-20 protein levels between wild-type and eat-2 mutant animals via immunoblots. The ACS-20::GFP::3 ✕ FLAG protein levels are mildly but significantly elevated under DR (Fig. 1g, h), suggesting ACS-20 is involved in the response to low nutrient conditions.
Temporospatial requirement of ACS-20 in DR-induced longevity
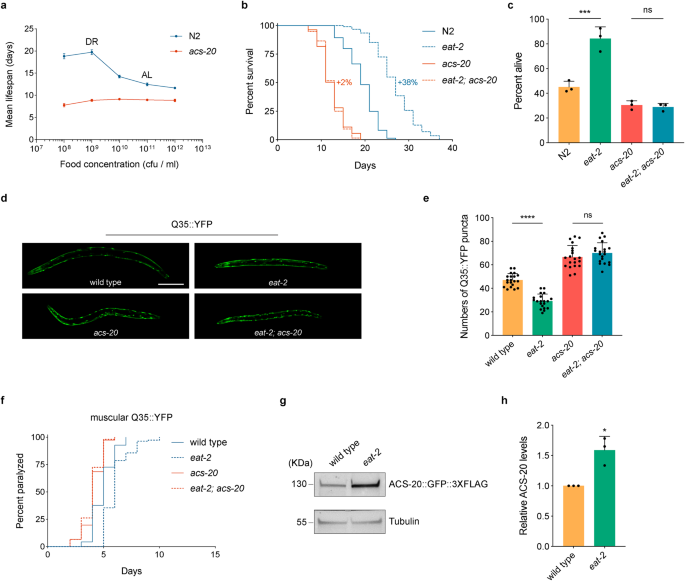
The acs-20::gfp::3 x flag knockin (KI) strain allows us to characterize the endogenous expression patterns of ACS-20. Confocal microscopy images show that ACS-20 is mainly expressed in the epidermal and muscular tissues (Fig. 2a), with the expression levels significantly diminished during adulthood (Fig. 2b). RT-qPCR measurement of the endogenous acs-20 mRNA levels confirms that acs-20 expression levels in adulthood are significantly lower than those in the third (L3) or fourth (L4) larval stages (Fig. 2c). Thus, acs-20 is expressed in a temporospatial manner.
a Representative photographs of the endogenous ACS-20::GFP::3✕FLAG expression in different tissues. Scale bar, 20 μm. b Representative photographs of ACS-20::GFP expression in the fourth larval stage (L4) and day 1 adulthood. Scale bar, 50 μm. c RT-qPCR quantification of acs-20 mRNAs at different stages. Data are represented as mean ± SD (n = 3). ns, p = 0.9880; ***p < 0.001 (One-way ANOVA with Turkey’s multiple comparisons test). d, e Survival curves of N2 and eat-2 mutant animals treated with the global control or acs-20 RNAi in whole life (d) or adulthood (e). N2 vs. eat-2 with the control RNAi treatment, p < 0.0001 (whole life, adulthood); N2 vs. eat-2 with the acs-20 RNAi treatment, p = 0.3093 (whole life), p < 0.0001 (adulthood) (log-rank tests). f, g Survival curves of wild type (wt) and eat-2 mutant animals treated with the epidermal (f) or muscular (g) control or acs-20 RNAi. wt vs. eat-2 with the control RNAi treatment, p < 0.0001 (epidermal RNAi, muscular RNAi); wt vs. eat-2 with the acs-20 RNAi treatment, p = 0.0662 (epidermal RNAi), p < 0.0001 (muscular RNAi) (log-rank tests). h Survival curves of acs-20 and eat-2; acs-20 mutants with or without the acs-20 transgene driven by the epidermis-specific dpy-7 promoter. acs-20 vs. eat-2; acs-20, p = 0.9773 (without the transgene), p < 0.0001 (with the transgene) (log-rank tests). Percentages indicate changes in mean lifespan induced by the eat-2 mutation. Source data are provided as a Source Data file.
To determine when and where ACS-20 functions to regulate lifespan under DR, we performed RNAi knockdown experiments to examine the effects of acs-20 reduced function on longevity. Consistent with the temporal expression patterns, the whole life acs-20 RNAi treatment completely abolishes DR-induced lifespan extension (Fig. 2d), whereas acs-20 RNAi only during adulthood has little effect on lifespan (Fig. 2e). Suppression of DR-induced longevity can also be achieved by the epidermis-specific acs-20 RNAi treatment (Fig. 2f), but not by muscle-specific acs-20 RNAi (Fig. 2g). To validate these findings, a single-copy transgene of acs-20 driven by its own promoter was constructed for rescue experiments. The DR-induced lifespan extension deficiency of acs-20 is fully restored by this transgene (Supplementary Fig. 2). Another single-copy transgene of acs-20 driven by the dpy-7 promoter, which is specifically active in the epidermis only during development26, also completely restored DR-induced longevity in the acs-20 mutant background (Fig. 2h). Collectively, ACS-20 functions in the epidermis during developmental stages to regulate DR-induced longevity.
Effects of acs-20 on lipid metabolism
ACS-20 is orthologous to the mammalian FATP4, which serves predominantly as an acyl-CoA synthetase rather than a fatty acid transporter27. Mutations in humans or mice FATP4 lead to lipid metabolic defects in the skin21,22. To characterize whether the acs-20 mutant affects lipid accumulation, we performed Oil Red O (ORO) staining to measure neutral lipid levels in the intestine, the major metabolic and lipid storage tissue in C. elegans. Imaging and quantification results demonstrate that the acs-20 mutation does not affect the intestinal lipid levels compared to the wild-type N2, whereas the eat-2; acs-20 mutant has slightly increased lipid accumulation compared to the eat-2 mutant (Supplementary Fig. 3a, b).
Since ACS-20 functions in the epidermis, another lipid storage tissue in C. elegans, to regulate lifespan, it is important to examine whether the acs-20 mutation affects lipid accumulation in the epidermis. However, the ORO staining method with fixed animals does not allow quantitative assessment of epidermal lipid droplets. Previous studies have shown that DGAT-2 is a membrane protein of lipid droplets, and a single-copy gfp::dgat-2 transgene driven by the intestine-specific vha-6 promoter can be used for quantitative measurement of intestinal lipid droplets28. We then constructed a single-copy gfp::dgat-2 transgene driven by the epidermis-specific col-12 promoter. Imaging and quantification assays show that the acs-20 mutation does not affect epidermal lipid droplet levels either in the wild type or in the eat-2 mutant background (Supplementary Fig. 3c, d). Altogether, these results suggest that ACS-20 might not regulate DR-induced lifespan extension via affecting lipid accumulation in metabolic tissues.
Functional transcriptomics analysis of the acs-20 mutant
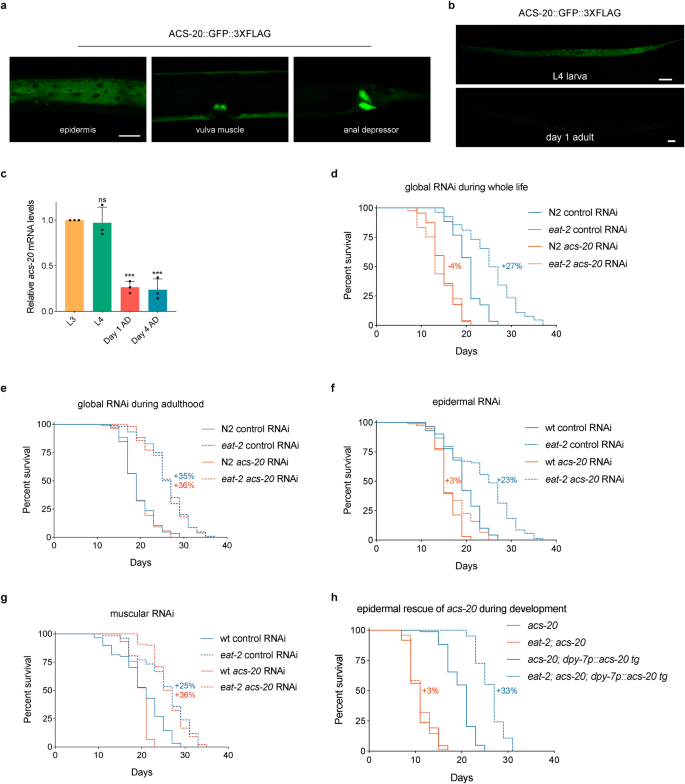
To gain mechanistic insights of ACS-20 for its role in ageing, we conducted transcriptional profiling of the wild-type N2, acs-20, eat-2, and eat-2; acs-20 mutant animals via mRNA-Seq. Bioinformatic analysis reveals that with the cutoff at fold changes >2 and adjusted p < 0.01, there are 61 upregulated and 45 down-regulated genes in acs-20 compared to N2, whereas there are 138 upregulated and 38 down-regulated genes in eat-2; acs-20 compared to eat-2 (Fig. 3a and Supplementary Data 1). The 95 genes that are significantly upregulated in eat-2; acs-20 compared to eat-2 but show no significant changes in acs-20 compared to N2 were used for Gene Ontology (GO) analysis as these genes are more likely to be involved in the DR-specific response (Fig. 3b). The most significantly enriched GO term is endoplasmic reticulum (ER) unfolded protein response (Fig. 3c).
a Volcano plots of differentially expressed genes in the acs-20 vs. N2 and eat-2; acs-20 vs. eat-2 data sets. Red and blue points represent genes with significantly increased or decreased expression, respectively (p < 0.01, Wald tests with Bonferroni corrections). b A Venn diagram comparing genes upregulated in eat-2; acs-20 vs. eat-2 and genes not differentially expressed in acs-20 vs. N2. c Significantly enriched Gene Ontology (GO) terms (hypergeometric tests with Benjamini-Hochberg corrections) for genes significantly upregulated in the eat-2; acs-20 mutant compared to the eat-2 mutant. d RT-qPCR quantification of abu genes in eat-2 and eat-2; acs-20 mutant animals. Data are represented as mean ± SD (n = 3). ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns, p = 0.0598 (two-tailed t-tests). e, f Representative photographs of the ER stress reporter hsp-4p::gfp expression in wild-type, acs-20, eat-2, and eat-2; acs-20 animals treated with DMSO or tunicamycin (e) and quantification of the GFP fluorescence intensities (a.u. arbitrary unites). Data are represented as mean ± SD (n = 10). ****p < 0.0001; ns, acs-20 p > 0.9999, eat-2; acs-20 p = 0.9993 (One-way ANOVA with Turkey’s multiple comparisons test). Scale bar, 200 μm. g Percentages of N2, acs-20, eat-2, and eat-2; acs-20 mutant animals that died (dead), developmentally arrested (L1-L3) or completed development (L4) upon 0, 0.25 or 0.50 μg/ml tunicamycin treatments. Data are represented as mean ± SD (n = 3). For animals that completed development upon tunicamycin treatment, N2 vs. acs-20, p < 0.01; eat-2 vs. eat-2; acs-20, p < 0.0001 (Two-way ANOVA with Turkey’s multiple comparisons test). Source data are provided as a Source Data file.
RT-qPCR experiments confirmed the mRNA-Seq results that mRNA levels of multiple ER stress-related abu genes are significantly elevated in the eat-2; acs-20 double mutant compared to the eat-2 mutant (Fig. 3d). abu genes encode endomembrane proteins, the expression of which is induced in ER-stressed animals when the IRE-1–XBP-1 ER unfolded protein response (ERUPR) pathway is inactivated29. Tunicamycin is a protein N-glycosylation inhibitor which induces ER stress30. The tunicamycin treatment leads to robust activation of the hsp-4::gfp reporter, which has been widely used to monitor the ERUPR activation in C. elegans. Intriguingly, tunicamycin cannot activate hsp-4::gfp expression when acs-20 is mutated either under normal feeding or DR conditions (Fig. 3e, f), suggesting the acs-20 mutant might have reduced capability to deal with ER stress. Consistently, tunicamycin treatments cause more severe developmental delay and lethality when acs-20 is deleted in the wild-type or eat-2 mutant background (Fig. 3g). Dithiothreitol (DTT) and thapsigargin are also ER stressors that function via affecting the ER lumen oxidative environment31 and causing ER Ca2+ depletion32, respectively. DTT or thapsigargin treatment leads to more severe developmental defects in the acs-20 and eat-2; acs-20 mutants compared to the wild-type N2 and eat-2 mutant, respectively (Supplementary Fig. 4). These results suggest that the acs-20 mutation might compromise proteostasis thus increasing the sensitivity to ER stress, which contributes to the lifespan and healthspan defects under DR.
PTR-8/Patched as a key mediator of acs-20 for its roles in longevity and proteostasis regulation under DR
It is plausible that genes upregulated in the eat-2; acs-20 double mutant are responsible for the lifespan and proteostasis defects of acs-20 under DR. Therefore, we performed an RNAi screen in the eat-2; acs-20 double mutant background to individually knock down the 95 genes that are specifically upregulated by the acs-20 mutant under DR for lifespan extension phenotypes. Knockdown of ptr-8, which encodes an ortholog of the Hedgehog receptor, significantly extends lifespan of the eat-2; acs-20 mutant. We then constructed a ptr-8 knockout (KO) mutant via CRISPR/Cas9. In the acs-20 mutant, DR induces significant lifespan extension in the absence of PTR-8 (ptr-8; acs-20 vs. ptr-8 eat-2; acs-20) compared to the controls (acs-20 vs. eat-2; acs-20), which show no significant DR-mediated changes in lifespan (Fig. 4a). In addition, RT-qPCR assays confirmed that compared to the eat-2 mutant, the ptr-8 mRNA levels are robustly elevated by nearly 10-fold in the eat-2; acs-20 double mutant, whereas the difference between N2 and acs-20 mutant is not significant (Fig. 4b). Furthermore, the ptr-8 deletion reduces mRNA levels of ER stress-related abu genes in eat-2; acs-20 compared to eat-2 (Fig. 4c), and significantly increases the resistance to various ER stressors including tunicamycin, DTT, and thapsigargin in the eat-2; acs-20 mutant (Fig. 4d and Supplementary Fig. 4).
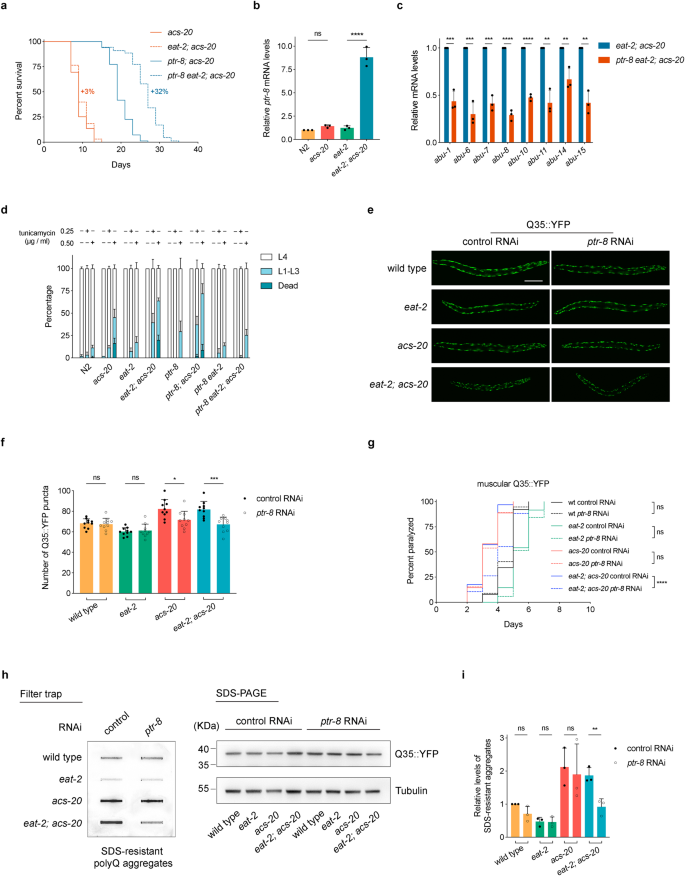
a Survival curves of acs-20, eat-2; acs-20, ptr-8; acs-20, and ptr-8 eat-2; acs-20. acs-20 vs. eat-2; acs-20, p = 0.0995; ptr-8; acs-20 vs. ptr-8 eat-2; acs-20, p < 0.0001 (log-rank tests). Percentages indicate changes in mean lifespan induced by eat-2. b RT-qPCR quantification of ptr-8 mRNAs in N2, acs-20, eat-2, and eat-2; acs-20. Data are represented as mean ± SD (n = 3). ****p < 0.0001; ns, p = 0.7886 (One-way ANOVA with Turkey’s multiple comparisons test). c RT-qPCR quantification of abu genes in eat-2; acs-20 and ptr-8 eat-2; acs-20. Data are represented as mean ± SD (n = 3). ****p < 0.0001; ***p < 0.001; **p < 0.01 (two-tailed t-tests). d Percentages of N2, acs-20, eat-2, eat-2; acs-20, ptr-8, ptr-8; acs-20, ptr-8 eat-2, and ptr-8 eat-2; acs-20 that died (dead), developmentally arrested (L1-L3) or completed development (L4) upon 0, 0.25 or 0.50 μg/ml tunicamycin treatment. Data are represented as mean ± SD (n = 3). For animals that completed development upon tunicamycin treatment, eat-2 vs. eat-2; acs-20, p < 0.0001; eat-2; acs-20 vs. ptr-8 eat-2; acs-20, p < 0.0001 (Two-way ANOVA with Turkey’s multiple comparisons test). e, f Representative photographs of wild-type, acs-20, eat-2, and eat-2; acs-20 animals expressing muscular Q35::YFP treated with the control or ptr-8 RNAi (e) and quantification of the Q35::YFP punctae (f). Data are represented as mean ± SD (n = 10). ****p < 0.0001; **p < 0.01 (One-way ANOVA with Turkey’s multiple comparisons test). Scale bar, 100 μm. g Paralysis analysis of the wild-type, eat-2, acs-20, and eat-2; acs-20 mutant animals expressing muscular Q35::YFP with control or ptr-8 RNAi. ****p < 0.0001; ns, p > 0.05 (log-rank tests). h, i Immuno-blots (h) and quantification (i) of the SDS-resistant Q35::YFP aggregates, total Q35::YFP, and tubulin in the wild-type, eat-2, acs-20, and eat-2; acs-20 with control or ptr-8 RNAi. Data are represented as mean ± SD (n = 3). **p < 0.01; ns, p > 0.05 (One-way ANOVA with Turkey’s multiple comparison tests). Source data are provided as a Source Data file.
Since the ptr-8 deletion significantly abrogates the ER stress response in eat-2; acs-20, we then set out to characterize the role of PTR-8 in proteostasis using the transgenic polyQ model. Imaging and quantification experiments show that compared to the control RNAi, the ptr-8 RNAi treatment significantly reduces the amount of the Q35::YFP aggregates in the eat-2; acs-20 double mutant (Fig. 4e, f). Consistently, the ptr-8 RNAi treatment also significantly delays age-related paralysis caused by the Q35::YFP aggregation (Fig. 4g). To make more quantitative assessment of DR, acs-20, and ptr-8 for their roles in proteostasis, we performed filter trap assays and immunoblots to measure aggregated and total Q35::YFP respectively in the wild-type, eat-2, acs-20, and eat-2; acs-20 animals treated with the control or ptr-8 RNAi (Fig. 4h). Quantification analysis shows that compared to the wild-type animals, the DR-mimicking eat-2 mutant has significantly less Q35::YFP aggregation, whereas this effect is abolished by the acs-20 mutation (Fig. 4i). On the other hand, inhibition of ptr-8 restores the protective effect of DR as indicated by significantly reduced levels of Q35::YFP aggregates in the eat-2; acs-20 mutant treated with the ptr-8 RNAi compared to the control RNAi (Fig. 4h, i). Altogether, these results demonstrate that PTR-8 functions downstream of acs-20 as a key regulator of proteostasis and longevity under DR.
NHR-23/RORA functions downstream of acs-20 to repress ptr-8 transcription
Since the ptr-8 mRNA levels are significantly elevated in the eat-2; acs-20 mutant, we constructed a reporter strain that carries a 3 kb ptr-8 promoter driving gfp transgene to study the transcriptional regulation mechanisms. This reporter strain shows GFP expression mainly in the epidermis, and the epidermal GFP signal is significantly increased in the eat-2; acs-20 mutant compared to the wild type (Fig. 5a). These expression patterns suggest that there might be either transcriptional repressors that restrict ptr-8 expression in the wild-type background, or transcriptional activators that promote ptr-8 expression in the eat-2; acs-20 mutant. To test these hypotheses, we performed genetic screens for altered ptr-8p::gfp expression using the transcription factor RNAi sub-library in the wild-type and eat-2; acs-20 mutant backgrounds, respectively. Although we did not find any RNAi treatment that reduces the high level of ptr-8p::gfp expression in eat-2; acs-20, we identified NHR-23 as a transcriptional repressor of ptr-8 in the wild type as RNAi knockdown of nhr-23 increases not only expression levels of the ptr-8p::gfp reporter (Fig. 5b), but also endogenous ptr-8 mRNA levels as quantified via RT-qPCR (Fig. 5c). nhr-23 encodes a nuclear hormone receptor that is orthologous to RORA, a key transcriptional regulator of the circadian clock33. Like acs-20, epidermis-specific RNAi knockdown of nhr-23 also significantly blocks DR-induced lifespan extension (Fig. 5d).
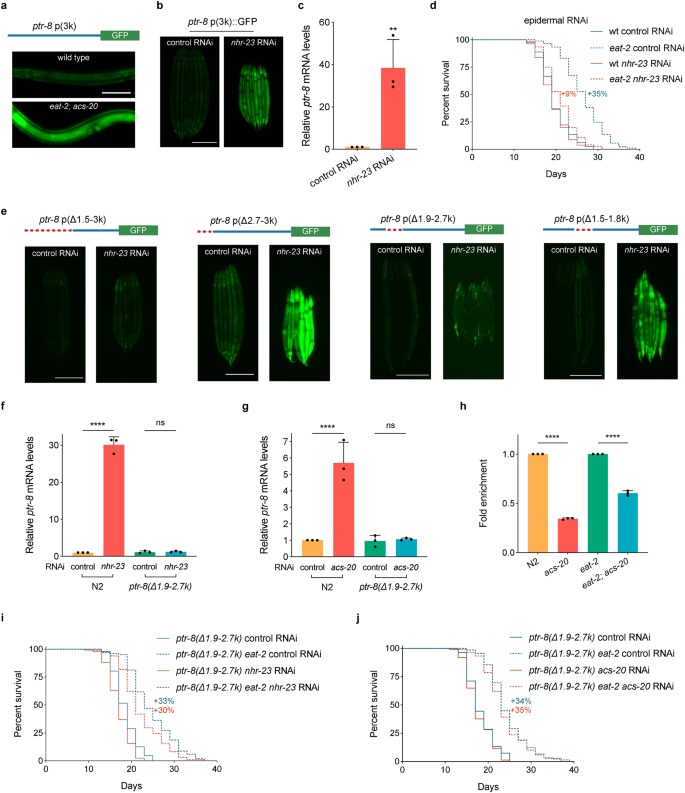
a Representative photographs of the ptr-8 p(3k)::GFP reporter expression in wild-type and eat-2; acs-20 animals. Scale bar, 50 μm. b Representative photographs of the ptr-8 p(3k)::GFP expression in animals treated with the control or nhr-23 RNAi. Scale bar, 200 μm. c RT-qPCR quantification of ptr-8 mRNA levels in wild-type animals treated with the control or nhr-23 RNAi. Data are represented as mean ± SD (n = 3). **p = 0.0084 (two-tailed t-test). d Survival curves of wild type (wt) and eat-2 mutant animals treated with the epidermis-specific control or nhr-23 RNAi. wt vs. eat-2: control RNAi, p < 0.0001; nhr-23 RNAi, p < 0.01 (log-rank tests). e Representative photographs of the ptr-8 p(Δ1.5-3 k)::GFP, ptr-8 p(Δ2.7-3 k)::GFP, ptr-8 p(Δ1.9-2.7 k)::GFP, and ptr-8 p(Δ1.5-1.8 k)::GFP expression in animals treated with the control or nhr-23 RNAi. Scale bar, 200 μm. f, g RT-qPCR quantification of ptr-8 mRNA levels in N2 and the ptr-8 p(Δ1.9-2.7 k) mutant animals treated with either control vs. nhr-23 RNAi (g) or control vs. acs-20 RNAi (h). Data are represented as mean ± SD (n = 3). ****p < 0.0001; ns, p = 0.9972 (nhr-23 RNAi), p = 0.9972 (acs-20 RNAi) (One-way ANOVA with Turkey’s multiple comparisons test). h ChIP-qPCR quantification of the relative enrichment of NHR-23::GFP binding with the ptr-8 promoter region (1861–1958 bp) in N2, acs-20, eat-2 and eat-2; acs-20. The fold enrichment was normalized by the input DNA. Data are represented as mean ± SD (n = 3). ****, p < 0.0001 (two-tailed t-tests). i, j Survival curves of ptr-8 p(Δ1.9-2.7 k) and ptr-8 p(Δ1.9-2.7 k) eat-2 mutant animals treated with the control vs. nhr-23 RNAi (i) or acs-20 RNAi (j). ptr-8 p(Δ1.9–2.7 k) vs. ptr-8 p(Δ1.9–2.7 k) eat-2: control RNAi, p < 0.0001; nhr-23 RNAi, p < 0.0001; acs-20 RNAi, p < 0.0001 (log-rank tests). Percentages indicate changes in mean lifespan induced by the eat-2 mutant. Source data are provided as a Source Data file.
To further characterize the transcriptional regulation of ptr-8 by NHR-23, we constructed transgenic lines carrying serial deletions of ptr-8 promoter::gfp reporters. In contrary to the 3 kb promoter, the ptr-8 p(Δ1.5-3k)::gfp reporter does not respond to nhr-23 RNAi knockdown (Fig. 5e), suggesting the key cis-regulatory element is located within the 1.5–3 kb region of the ptr-8 promoter. Further studies narrow the cis-regulatory element down to the 1.9–2.7 kb region of the ptr-8 promoter (Fig. 5e). We then set out to investigate whether the identified promoter region is functional regarding to the regulation of ptr-8 transcription and longevity in vivo. CRISPR/Cas9-based genome editing was performed to create a 833-bp deletion mutant of the 1.9–2.7 kb ptr-8 promoter region. RT-qPCR experiments reveal that the significantly elevated ptr-8 expression upon nhr-23 or acs-20 RNAi treatment is completely blocked in the absence of this promoter region (Fig. 5f, g). To examine whether NHR-23 interacts with the ptr-8 promoter region, we performed genome editing experiment to create the nhr-23::gfp knockin strain. ChIP-qPCR using anti-GFP antibodies show that NHR-23::GFP has reduced interaction with the ptr-8 promoter in the absence of ACS-20 (Fig. 5h). Moreover, deletion of the 1.9–2.7 kb ptr-8 promoter region is sufficient to restore DR-induced lifespan extension when animals are subjected to nhr-23 or acs-20 RNAi knockdown (Fig. 5i, j).
NHR-23 does not seem to be the only ACS-20 downstream transcriptional regulator of ptr-8 since deletion of the 1.9–2.7 kb ptr-8 promoter region is not sufficient to increase ptr-8 expression (Fig. 5e–g) or block DR-induced lifespan expression (Fig. 5i, j). One plausible model is that NHR-23 functions through inhibiting a transcriptional activator of ptr-8 rather than directly represses ptr-8 expression (Fig. 6). When the cis-regulatory element is deleted, neither NHR-23 or the unknown factor can affect ptr-8 expression. Animals thus show normal response to DR. Taken together, these results demonstrate that NHR-23 functions downstream of ACS-20 to repress ptr-8 expression and ensure DR-induced lifespan extension.
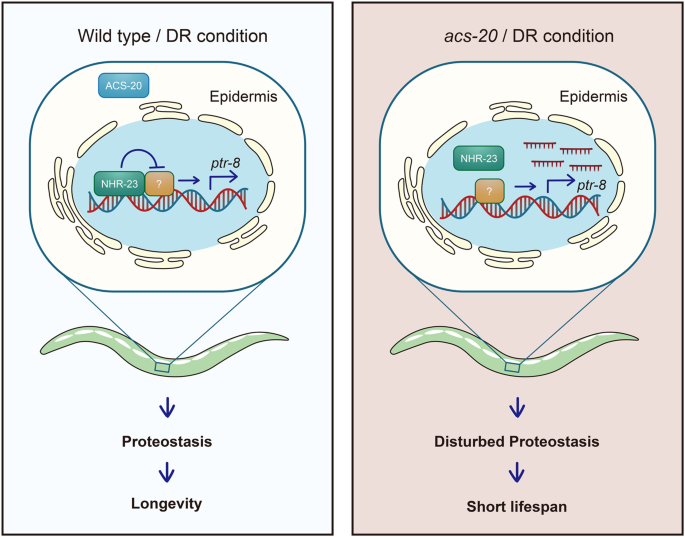
ACS-20 functions in the epidermis during development to regulate DR-induced lifespan extension. In the presence of ACS-20, the NHR-23/RORA nuclear hormone receptor functions with an unknown factor to restrict the expression of PTR-8/Patched via a cis-regulatory element to maintain proteostasis and promote healthy ageing under DR. In the absence of ACS-20, NHR-23-mediated transcriptional repression of ptr-8 is abolished. Overexpression of PTR-8 results in disrupted proteostasis and elevated ER stress, which block the lifespan extension produced by DR.
In summary, we have identified ACS-20/FATP4 as a key mediator of DR on healthy ageing from a targeted genetic screen. ACS-20 functions in the epidermis during development to regulate DR-induced lifespan extension. Under DR, ACS-20 functions through the NHR-23/RORA nuclear hormone receptor and potentially another transcriptional regulator via a cis-regulatory element in the ptr-8 promoter to repress its expression, thus ensuring proteostasis and prolonged longevity (Fig. 6). These findings reveal the key role of a lipid metabolic enzyme in nutrients-mediated modulation of ageing and elucidate the underlying transcriptional regulation mechanisms.