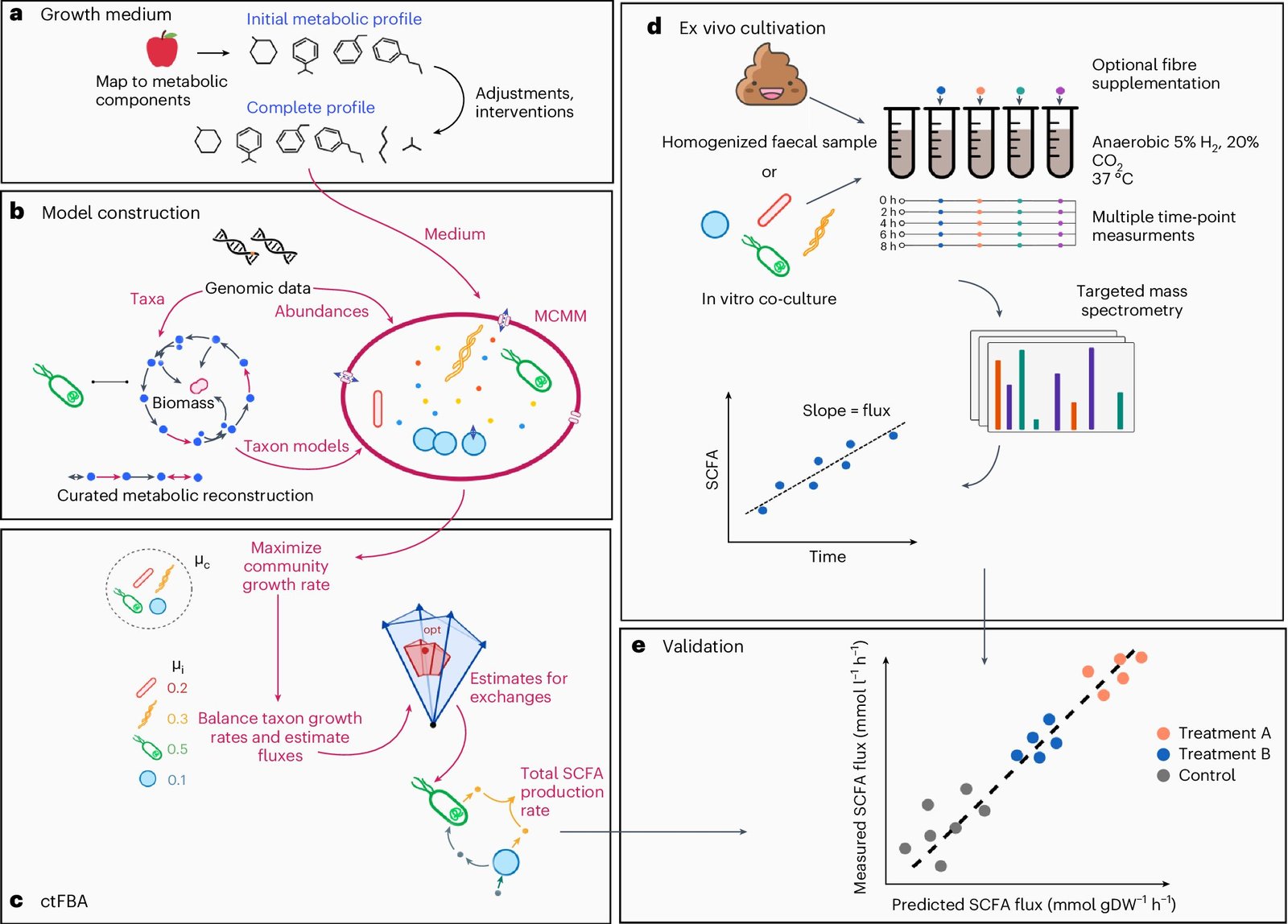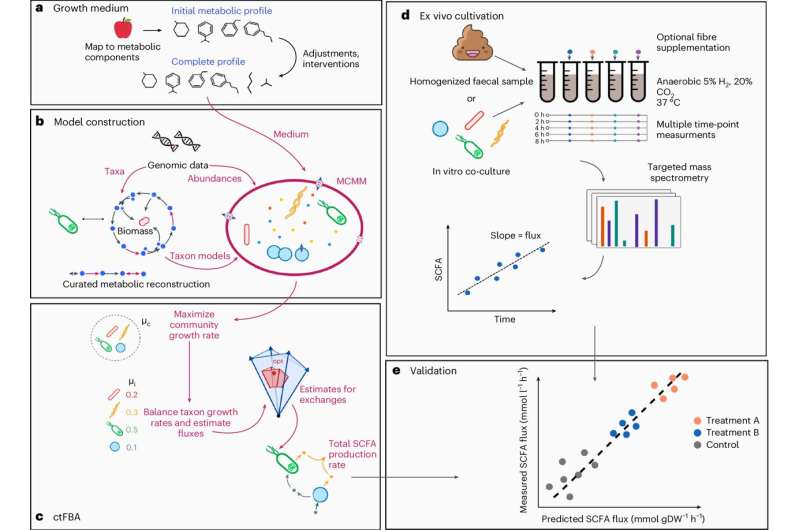
MCMM predicts personalized SCFA production profiles. Credit: Nature Microbiology (2024). Publication date: 10.1038/s41564-024-01728-4
Short-chain fatty acids (SCFAs) are beneficial molecules produced by gut bacteria and have been implicated in improving host metabolism, reducing systemic inflammation, improving cardiovascular health, and reducing cancer risk. However, SCFA profiles can vary significantly between individuals consuming the exact same diet, and currently we lack tools to predict these inter-individual differences.
Researchers at the Institute for Systems Biology (ISB) have developed a new method to simulate individualized microbiome-mediated responses to diet: they use a microbial community-scale metabolic modeling (MCMM) approach to predict individual-specific SCFA production rates in response to different dietary, prebiotic and probiotic intakes.
In other words, ISB scientists can use gut microbiome sequence data and information about dietary intake to build a “digital twin” of gut microbiome metabolism that can constrain each individual’s unique model and simulate their individual response to diet. They report the results in Nature Microbiology.
“Broadly speaking, the gut microbiome is a bioreactor that converts dietary fiber into these SCFAs,” said ISB Associate Professor and co-senior author Sean Gibbons, PhD. “Understanding how to quantitatively map the gut environment and dietary intake to SCFA output will be a major advance in translating microbiome science into the clinic.”
Unlike black-box machine learning predictive approaches, MCMMs are transparent and mechanistic, with tens of thousands of metabolites and enzymes across dozens of organisms providing advanced knowledge of the specific microbes, dietary components, and metabolic pathways that contribute to SCFA production. Despite this transparency, the complexity of these models makes them difficult to validate experimentally.
One approach is to measure SCFA production rates across ecosystems and compare these ecosystem-scale measurements with corresponding model predictions. However, measuring SCFAs in the wild is difficult because SCFAs are rapidly consumed in the body after they are produced.
To overcome this challenge, the authors measured SCFA production rates from in vitro populations of a random mixture of human gut bacterial isolates and from different human ex vivo fecal homogenates cultured in an anaerobic chamber containing different dietary fibers.
By separating microbial SCFA production from host absorption, the ISB scientists were able to show that for both butyrate and propionate, two of the most abundant and physiologically potent SCFAs, MCMM predictions correlated significantly with measured production rates across a range of fibres.
Although it was not possible to measure butyrate and propionate production in vivo, the authors were able to use indirect associations between SCFA production rates and blood-based health markers to examine the physiological consequences of individual differences in production.
First, we showed that MCMM predictions could distinguish between individuals with different immune responses in the high-fiber diet study. Most individuals showed a decrease in systemic markers of inflammation, but some showed an increase in inflammation on the high-fiber diet. MCMM predictions showed that individuals in the high inflammatory response group had a significantly reduced ability to produce propionate.
Next, the authors showed that butyrate predictions were significantly associated with blood markers of cardiometabolic and immune health in a population of over 2,000 individuals. Specifically, higher MCMM-predicted butyrate production was significantly associated with lower LDL cholesterol levels, lower triglyceride levels, improved insulin sensitivity, lower systemic inflammation, and lower blood pressure.
“The predictive accuracy of MCMM in vitro and the significant associations of SCFA predictions with health markers in human cohorts give us confidence in the utility of these models for precision nutrition,” said lead author Nick Quinn Bowman, PhD, a graduate student at Washington University ISB who recently published the paper.
After validating the MCMM predictions with various methods, the authors demonstrated the potential of this approach for designing personalized prebiotic, probiotic, and dietary interventions that optimize SCFA production profiles.
The researchers simulated butyrate production rates in a population of over 2,000 people from the Pacific West of the United States under two different diets: a standard Austrian diet (i.e. a standard European diet) and a vegan high-fiber diet.
The researchers found that a small number of participants showed little to no increase in butyrate production when they switched to a high-fiber diet (called “non-responders”), while other participants showed a slight decrease in butyrate production on a high-fiber diet (called “regressors”).
Next, the researchers simulated three simple co-interventions to boost butyrate production in non-responders and regressors: adding the prebiotic fiber inulin, adding the prebiotic fiber pectin, or adding a butyrate-producing probiotic (Faecalibacterium) to both background diets.
The results showed that there was no single combination intervention that was optimal for all individuals: some benefited most from adding prebiotic fiber, while others appeared to need to add a butyrate-producing probiotic to their microbiota.
“These results provide an important proof of concept for new avenues of microbiome-mediated precision nutrition,” said co-senior author Christian Diener, PhD, assistant professor at the Medical University of Graz in Austria.
“But of course, more work is needed to validate the predictive capabilities of these models in prospective human trials before they can enter clinical practice.”
For more information:
Nick Quinn-Bohmann et al. “Microbial community-scale metabolic model predicts short-chain fatty acid production profiles in the human gut.” Nature Microbiology (2024). Publication date: 10.1038/s41564-024-01728-4
Provided by the Institute for Systems Biology
Quote: A new path to microbiome-based precision nutrition (June 24, 2024) Retrieved June 24, 2024, from https://medicalxpress.com/news/2024-06-path-microbiome-precision-nutrition.html
This document is subject to copyright. It may not be reproduced without written permission, except for fair dealing for the purposes of personal study or research. The content is provided for informational purposes only.