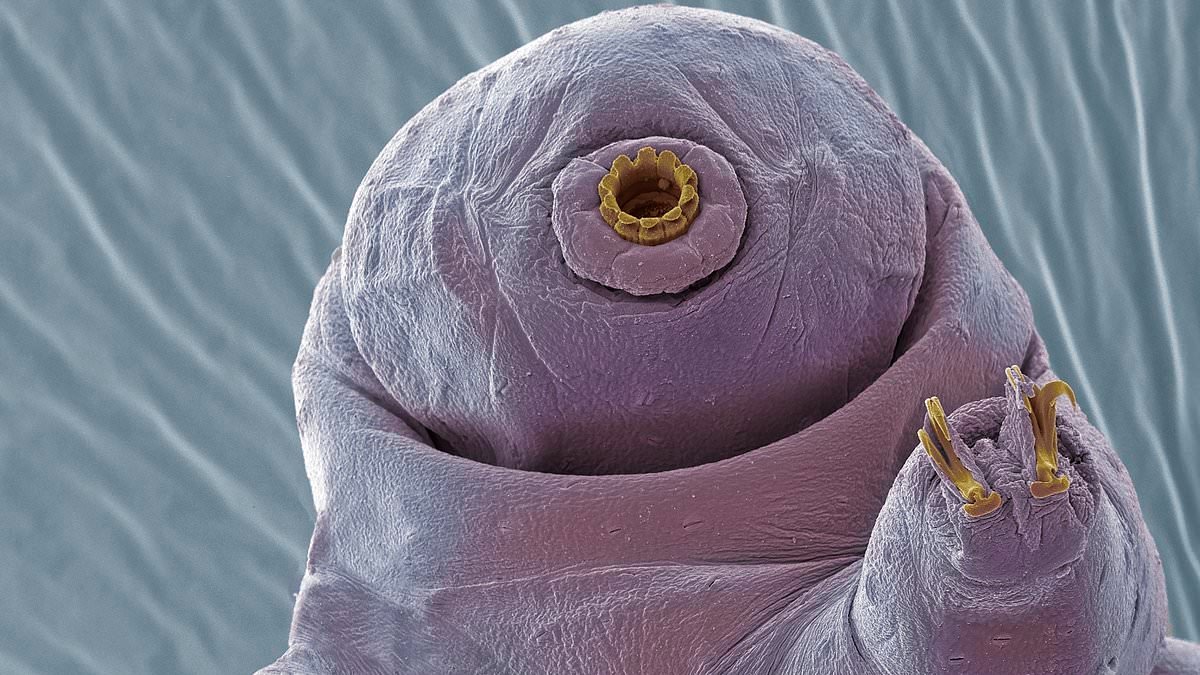
- When tardigrades are stressed, they enter a state of hibernation called biostasis.
- Proteins that enable biostasis also significantly slowed down human cells
- Scientists suspect tardigrade proteins could be used to slow cell death in humans
- Read more: Tardigrades suffocate on snail mucus but ride on top of their shells
Scientists studying mysterious creatures called tardigrades may have discovered a new potential tool for slowing aging in humans.
Also called tardigrades, these nearly microscopic eight-legged animals can survive conditions that would kill most other life forms.
That’s because it contains proteins that form gels inside cells, slowing life processes and reducing cell damage, which could become an anti-aging panacea.
When an international team of researchers introduced tardigrade proteins into human cells in the lab, they found that the cells became sluggish and entered a kind of hibernation, just like cells in “indestructible” organisms. discovered.
The new findings provide clues as to how human cells and tissues can be put into suspended animation, similar to tardigrades under stress.
Only two-hundredths of an inch long, tardigrades can withstand the immense pressures of the deep ocean, extreme heat and cold, complete dehydration, prolonged starvation, and even the vacuum and radiation of outer space.
Their secret: suspended animation.
When tardigrades are stressed, their entire body begins to slow down, including on a microscopic level.
They can enter a state called biostasis, where they can endure near complete dehydration for years until water becomes available again.
Now, scientists have discovered that a protein that enables homeostasis in tardigrades may have a similar effect on human cells.
“Surprisingly, when you introduce these proteins into human cells, they gel, just like tardigrades do, and slow down their metabolism,” said Sylvia Sánchez Martinez, a senior research scientist at the University of Wyoming.
“Furthermore, just like tardigrades, putting human cells with these proteins into biostasis makes them more resistant to stress, giving human cells some of the tardigrade’s abilities.”
This discovery could mean tardigrades are an important weapon in the fight against aging in humans.
If our cells could resist DNA damage from exposure to the sun and toxic substances like tardigrade cells, perhaps the entire aging process could be slowed down, suggest scientists behind a new study. did.
More directly, this discovery means that medicines such as human stem cells and hemophilia blood products that require refrigeration can be transported without them, increasing access to life-saving medicines for people in developing countries. means expanding.
A key part of this process is a series of proteins called CAHS, which are “intrinsically disordered” proteins that slow the tardigrade until it reaches bioquiescence.
In biostasis, the animal turns into a “tun”, the name of the dormant tardigrade.
This ability is one of many that allows you to survive extreme conditions.
In Tun State, tardigrades are unharmed by all kinds of abuse.
They can be exposed to radiation many times the amount that would kill humans, but damage suppressors in their DNA keep them safe.
It can heat up to 300 degrees Fahrenheit or cool down to nearly 500 degrees below freezing.
As long as they are in a biostatic state, they are safe.
Scientists thought this was due to a sugar called trehalose that tardigrades and other dehydration-tolerant microorganisms can produce.
But recently it became clear that that’s not the whole story.
While trehalose appears to protect some sensitive biological substances, the CAHS protein appears to be responsible for slowing everything down and stopping the tardigrades in time.
In biostasis, the fluid in the tardigrade’s body turns into a gel as the molecules slow down and enter a tun state.
Previous studies have shown that when a tardigrade begins bioquiescence, its body produces more and more CAHS proteins.
And the more CAHS protein there is, the more gel-like the animal’s interior becomes.
In the new study, introducing the CAHS protein into human cells also slowed the cells down and caused them to gel.
“Surprisingly, when you introduce these proteins into human cells, they gel and slow down their metabolism, just like tardigrades do,” said study author Sylvia Sánchez Martinez, a senior research scientist at the University of Wyoming.
“Furthermore, just like tardigrades, when human cells with these proteins are put into biostasis, they become more resistant to stress, giving human cells some of the tardigrade’s abilities.” Martinez said.
Additionally, this process can be reversed, just as tardigrades break out of biostasis.
“When stress is relieved, the tardigrade gel dissolves and human cells return to normal metabolism,” said lead author Thomas Boothby, assistant professor of molecular biology at the University of Wyoming.
The results provide insight into how tardigrades develop such ethereal stress tolerance, but they go beyond that, Boothby, Sánchez-Martinez, and their co-authors say. They write as follows: Whole organisms can also slow down aging and increase storage and stability. ”
The study was published in the journal Protein Science.