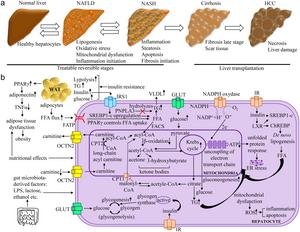
image:
The pathogenesis of NAFLD is multifactorial and involves several molecular pathways. High FFA flux generated by WAT led to functional regulation of hepatocytes by decreasing β-oxidation and increasing Krebs cycle and gluconeogenesis. This leads to reduced ATP production, mitochondrial dysfunction, and ER stress. In this context, high TG and VLDL signatures indicate the pathophysiological state of the disease. Insulin stress reduces glycogenogenesis and Newly Lipogenesis in hepatocytes. Genetic variants in PNPLA3 affect hepatic TG hydrolysis. Less carnitine biosynthesis leads to less transport of fatty acids to mitochondria for β-oxidation. High insulin levels promoted upregulation of SREBP1-c, increasing fatty acids in hepatocytes. When electron transport chain uncoupling occurred, superoxide generated by NADPH oxidase induced liver injury. In addition, gut resident bacteria-released products such as LPS, lactose, and ethanol contributed to the pathogenesis of NAFLD. The symbols “↑”, “↓”, and “┴” represent “high”, “low”, and “inhibition”, respectively. ATP, adenosine triphosphate. ChREBP, carbohydrate response element-binding protein. CPT2, carnitine palmitoyltransferase 2.−electron; ER, endoplasmic reticulum; FATP, fatty acid transport protein 1; FFA, free fatty acid; GLUT, glucose transporter; HCC, hepatocellular carcinoma; IR, insulin receptor; IRS, insulin receptor substrate; IRS1, insulin receptor substrate 1; LPS, lipopolysaccharide; LXR, liver X receptor; NADP, nicotinamide adenine dinucleotide phosphate; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PNPLA3, patatin-like phospholipase domain-containing protein 3; OCTN2, organic cation transporter; PPARγ, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; SREBP1c, sterol regulatory element-binding protein 1; TG, triglycerides; TNFα, tumor necrosis factor alpha; WAT, white adipose tissue; VLDL, very low density lipoprotein.
View more
Credit: Suvendu Sarkar
Nonalcoholic fatty liver disease (NAFLD) is an emerging global health problem that is recognized as one of the leading causes of chronic liver disease. The global prevalence of NAFLD is 32.4%, with a higher prevalence in men (39.7%) than women (25.6%). NAFLD is characterized by abnormal accumulation of lipids in hepatocytes and elevated liver enzyme levels. Progression of NAFLD can lead to nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC). Recently, NAFLD has been redefined as metabolic dysfunction-associated fatty liver disease (MAFLD) to better reflect its association with metabolic abnormalities. MAFLD is associated with a higher risk of disease progression compared to NAFLD.
Vitamin D, an important fat-soluble vitamin, plays a key role in calcium homeostasis and bone health. Beyond these classic roles, vitamin D is known to have potential benefits in various non-bone health domains, including anti-inflammatory, antioxidant and anti-fibrotic properties. The liver is essential for vitamin D metabolism, converting it into its active form, calcitriol, which exerts a variety of biological effects.
Mechanistic insights into the role of vitamin D in NAFLD
Anti-inflammatory Effects: Vitamin D modulates the immune response by downregulating pro-inflammatory cytokines such as TNF-α and IL-6 and upregulating anti-inflammatory cytokines such as IL-10. This modulation helps reduce liver inflammation, a key component of NAFLD progression.
antioxidant effect: Vitamin D promotes the expression of antioxidant enzymes and reduces oxidative stress in hepatocytes, and this antioxidant effect is particularly valuable because oxidative stress is the main cause of liver damage in NAFLD.
Lipid metabolism: Vitamin D affects lipid metabolism by regulating the expression of genes involved in fatty acid synthesis and oxidation. Vitamin D promotes lipid oxidation and reduces lipid synthesis, helping to prevent lipid accumulation in the liver.
Anti-fibrosis: Vitamin D inhibits the activation of hepatic stellate cells, which are crucial for the development of liver fibrosis, and this inhibition occurs through various signaling pathways, suggesting that vitamin D may have a protective effect against liver fibrosis.
Numerous clinical studies and trials have investigated the relationship between vitamin D levels and NAFLD. Observational studies have consistently shown an inverse correlation between serum vitamin D levels and the severity of NAFLD. Randomized controlled trials have demonstrated that vitamin D supplementation results in significant improvements in liver enzyme levels, reductions in liver fat content, and improvements in the histological features of NAFLD. These results highlight the potential of vitamin D as a therapeutic agent in the management of NAFLD.
The therapeutic effects of vitamin D in NAFLD management are promising due to the multifaceted roles of vitamin D on liver health. However, the optimal dosage, duration of treatment, and long-term effects of vitamin D supplementation have yet to be fully elucidated. In addition, the interactions of vitamin D with other NAFLD treatments, such as lifestyle modifications and medications, require further investigation. Future studies should focus on large-scale, long-term clinical trials to confirm the benefits of vitamin D and establish comprehensive guidelines for vitamin D supplementation in NAFLD management.
Vitamin D has anti-inflammatory, antioxidant, and anti-fibrotic properties, making it a useful adjunctive therapy for NAFLD. Further research is needed to establish clear guidelines for vitamin D supplementation in NAFLD and optimize patient outcomes. As research progresses, vitamin D may become the cornerstone of a multifaceted approach to managing NAFLD and its complications.
Full text:
https://www.xiahepublishing.com/2472-0712/ERHM-2023-00019
This study was recently Exploratory research and hypotheses in medicine.
Exploratory research and hypotheses in medicine (ERHM) publishes original exploratory research articles and cutting-edge reviews that focus on new discoveries and the latest scientific advances that support new hypotheses in medicine. The journal accepts a wide range of topics, including innovative diagnostic and treatment modalities and insightful theories relevant to medical practice. Exploratory studies published in ERHM do not necessarily need to be comprehensive and conclusive, but the study design must be sound, the methodology reliable, the results bona fide, and the hypotheses reasonable and justifiable by evidence.
Follow X: translation:
Follow us on LinkedIn: Natsuwa Publishing Co., Ltd.
Disclaimer: Neither AAAS nor EurekAlert! are responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for any use of information provided through the EurekAlert system.