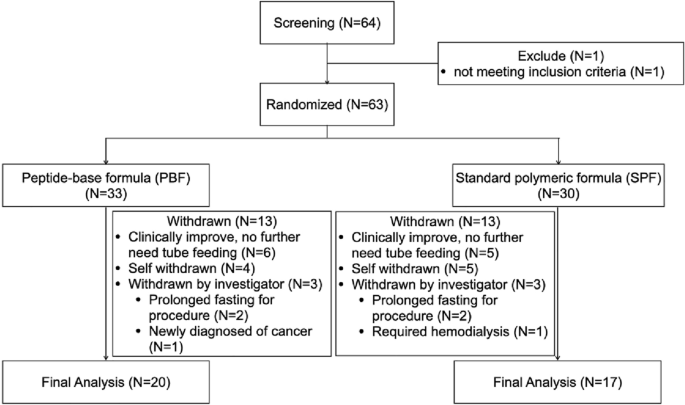
Study design
This prospective, randomized, double-blind, multicenter study was conducted between January 2019 and June 2022 in the ICUs and Respiratory Care Units (RCUs) of Songkhla Nagarind Hospital, Songkhla, Phramongkutklao Hospital, Bangkok, and Vajira Hospital, Bangkok.
Study population
All mechanically ventilated patients admitted to the ICU or RCU during the study period (January 2019 to June 2022) were screened based on the inclusion criteria: (1) age ≥18 years, (2) body mass index (BMI) 18–30 kg/m2(3) The patient is expected to require mechanical ventilation for more than two days; (4) The patient is at risk of malnutrition and requires nutritional support. [based on mNUTRIC Score ≥ 5 points](5) The patient must be hemodynamically stable and receive enteral nutrition via a nasogastric tube within 48 hours of admission to the ICU; and (6) the patient is expected to receive nutrition solely via a nasogastric tube for 5 days or more.
The following patients were excluded: (1) Patients at risk for or with a history of aspiration pneumonia. (2) Patients with thyroid disease. (3) Patients with severe liver dysfunction (Child–Pugh score, class C). (4) Patients receiving renal replacement therapy. (5) Patients with suspected or diagnosed abdominal hypertension. (6) Patients with HIV infection or autoimmune disease. (7) Patients with uncontrolled cancer or terminal illness. (8) Patients who had received chemotherapy or radiation therapy within the past 6 months. (9) Patients who had received immunosuppressants continuously for ≥2 weeks within the 4 months prior to ICU admission. (10) Patients with a history of hypersensitivity to any component of the nutritional formula. (11) Patients who required additional caloric and nutrient supplementation (e.g., parenteral nutrition) in addition to nasogastric EN to achieve caloric goals during the study period. Additionally, patients requiring fentanyl >2 μg/kg/h, morphine >0.05 mg/kg/h, or norepinephrine >0.3 μg/kg/min were excluded.
Ethical approval
The study protocol was developed in accordance with the ethical principles outlined in the Declaration of Helsinki and the ICH Good Clinical Practice guidelines for medical research involving human subjects. The study protocol and informed consent forms were approved by all participating institutions, including the Human Research Ethics Committee (HREC) of the Faculty of Medicine, Prince of Songkla University (REC.61–124-14–1), the Institutional Review Board (IRB) of the Royal Thai Army Medical Department (IRB number P003h/61), and the Research Ethics Committee of the Faculty of Medicine, Vajira Hospital, Nabamindrajiraj University (IRB number 036/61).
This study was registered with the Thailand Clinical Trials Registry (registration number TCTR20220221006). All participants or their legal representatives provided written informed consent before enrollment in the study.
Study Protocol
Block randomization was performed via a funding body-sponsored computer system, with stratification by study site location. Participants were allocated in equal numbers to either PBF or SPF. Following patient recruitment and randomization, each study site accessed the randomization sequence via a secure telephone system. The study was designed to be double-blind, so all parties, including treating clinicians, caregivers, participants, researchers, and sponsors, were blinded to the specific enteral formula administered throughout the study period. Formulas were provided as powders labeled “Product A” or “Product B” and were purposely packaged indistinguishably to preserve the integrity of the blinding. These powders were prepared by hospital dietitians or nurses with a caloric density of 1 kcal/mL. The comprehensive biochemical composition of PBF and SPF is shown in Table 1.
Based on estimated or actual body weight, target energy requirements were set at 25 kcal/kg/day for the first 3 days after randomization and then increased to 30 kcal/kg/day from days 4 to 14 or until the end of the study period. No alternative nutritional or dietary supplements were allowed for participants during the study.
Enteral Feeding Protocol
Enteral nutrition (EN) was administered by continuous infusion starting at an initial rate of 20 mL/h, with enteral nutrients delivered by an infusion pump through a nasogastric tube. Gastric residual volume (GRV) was manually aspirated every 6 hours to monitor for signs of feeding intolerance. If GRV was less than 250 mL, the infusion rate was increased by 20 mL/h every 6 hours until the prescribed caloric intake was achieved. Conversely, for patients showing high GRV >250 mL, the infusion rate was halved or maintained at a minimum rate of 20 mL/h and metoclopramide 10 mg was administered intravenously every 6–8 hours. This regimen was continued until GRV levels declined to an acceptable threshold, after which the rate of EN was gradually increased towards the target rate. It is noteworthy that intravenous metoclopramide was the conventional treatment to manage elevated GRV in critically ill patients showing feeding intolerance, as intravenous erythromycin was not available in the country. This treatment protocol was maintained throughout the duration of the study.
Data collection
At enrolment, demographic data including age, sex, underlying diseases, reason for admission, weight and height were recorded. Biochemical parameters such as blood urea nitrogen (BUN) and creatinine (Cr), liver function tests, blood electrolyte levels, complete blood count (CBC) and coagulation contrast studies were performed. Biochemical tests were used to assess nutritional status including measurements of prealbumin, retinol binding protein, albumin and 24-h urinary nitrogen. In addition, levels of inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6) and procalcitonin were determined. Severity of illness was assessed at ICU admission using Acute Physiology and Chronic Health Evaluation II (APACHE II) and Simplified Organ Failure Assessment (SOFA) scores.
The Bhumibol Adulyadej Hospital Nutrition Triage (BNT) was employed for subjective assessment of patients’ nutritional status. The BNT incorporates aspects such as caloric intake history, unintentional weight loss, signs of edema, examination of subcutaneous fat and muscle loss, functional status, and the presence of acute, subacute, and chronic diseases that may cause inflammatory processes and metabolic abnormalities, making it a valid tool for nutritional assessment of adult patients in Thailand. Based on the overall BNT score, nutritional status can be classified into four strata: NT-1 (no risk of malnutrition, 0–4 points), NT-2 (mild malnutrition, 5–7 points), NT-3 (moderate malnutrition, 8–10 points), and NT-4 (severe malnutrition, >10 points).26.
Clinical and BNT assessments were performed daily. Total daily caloric and protein supplementation, and total and mean daily GRV were recorded. Biochemical parameters including CBC, serum electrolytes, liver and kidney function tests (BUN and Cr), retinol binding protein, prealbumin, albumin, CRP, procalcitonin, and IL-6 levels were analyzed at baseline and every other day for 14 days.
Twenty-four-hour urine collections for nitrogen and creatinine measurements were performed on days 1, 3, 5, and 14. Nitrogen balance was calculated using standard formulas previously described.27.
Safety Profile
All signs and symptoms of gastrointestinal intolerance, including vomiting, diarrhea, constipation, abdominal distension, and aspiration, were monitored daily by the investigators and recorded as adverse events (AEs). Diarrhea was assessed using the Hart-Dobb score, which is based on stool consistency and volume; a score of 12 or higher indicates diarrhea.28.
The incidence of diarrhea was calculated by the number of days that the patient had a Hart-Dobb score ≥12 divided by the patient’s duration of study participation (in days). All AEs were recorded in the AE report form, and the investigators adjusted for the relationship of these events to study equations. AEs were continuously monitored and followed during the study and for 28 days after the end of the study. These records were notified to the Institutional Ethics Committee according to standard regulations.
research result
The primary endpoint of the study was the time required to meet the established calorie goal between both enteral formulations. Predefined secondary endpoints included mean daily GRV, administration rate and total dose of intravenous metoclopramide as prokinetic therapy, days on mechanical ventilation, incidence of ICU-emergent infections, length of ICU stay, mortality in the ICU, and changes in serum markers of inflammation and nutritional status. Additionally, the study monitored the frequency of adverse events (AEs) and occurrence of diarrhea within each group.
Statistical analysis
A previous study in patients with acute gastrointestinal disorders observed a significant difference of 31 hours in time to achieve calorie goals between the PBF and SPF groups.29 Based on this discrepancy, we calculated the sample size: the sample size was designed to have a type I error (alpha) of 0.1 and a type II error (beta) of 0.2, requiring 39 participants in each group.
Continuous variables were presented as mean and standard deviation (SD), and categorical variables were expressed as frequency and percentage. Between-group differences in patient characteristics and outcomes of interest were evaluated using Student’s tPerform the α test, Wilcoxon rank sum test, Fisher’s exact test, and chi-square test as appropriate.
In repeated measures analyses, between-group comparisons of variables were performed using generalized estimating equations (GEE) with population-averaged models. Working correlation matrices for GEE analyses were selected based on lowest quasi-likelihood based on the independent model criterion (QIC). Interactions between group and time were quantified as β coefficients with 95% Wald confidence intervals (CIs).
The proportion of patients who achieved calorie goals was drawn using Kaplan-Meier curves and compared between groups by log-rank test. Regarding nutritional status determined daily by BNT scores, BNT scores were classified into four strata as previously described. Pairwise comparisons were then made within groups at three time intervals: days 1 and 7, days 1 and 14, and days 7 and 14. These comparisons were aimed at determining changes in BNT score strata using chi-square tests. Furthermore, pairwise comparisons of BNT score classifications on days 1, 7, and 14 were analyzed using chi-square tests to assess differences between groups.
Data were analysed on an intention-to-treat basis. Missing data were not imputed. PA mean score of less than 0.05 was considered statistically significant. Data analysis was performed using IBM SPSS Statistics for Macintosh (version 27.0, IBM Corp., Armonk, NY, USA) and MedCalc® Statistical Software, version 22.017 (MedCalc Software Ltd, Ostend, Belgium, https://www.medcalc.org, 2024).